Back to the Suture: The Distribution of Intraspecific Genetic Diversity in and Around Anatolia
Abstract
:1. Introduction
2. Phylogeographic Patterns
3. Patterns and Representative Case Studies
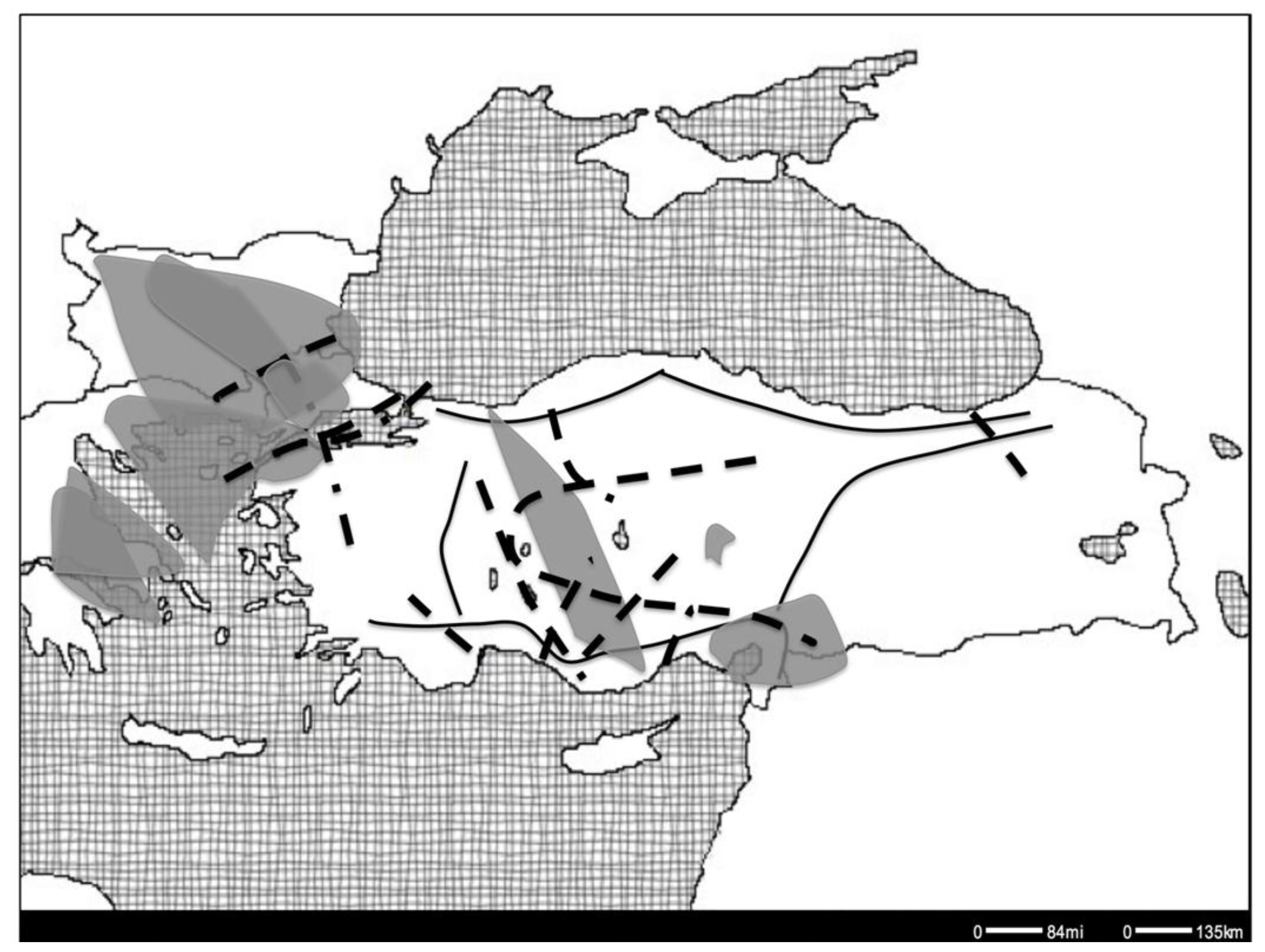
3.1. Pattern I: Anatolian-Balkan Suture Zone
3.2. Long-Fingered Bat (Myotis capacinnii)
3.3. Killifish (Aphanius fasciatus)
4. Pattern II: Intra-Anatolian Suture Zone
4.1. Ground Squirrels (Spermophilus spp.)
4.2. Bent-winged Bat (Miniopterus schreibersii)
5. The Star (*) Pattern
5.1. Oak-gallwasp (Andricus quercustozae)
5.2. Alpine Rockcress (Arabis alpina)
6. Special Cases
7. Discussion
7.1. Suture Zones
7.2. Postglacial Expansion Scenarios
7.3. Geographic Barriers
7.4. Times of Divergence
7.5. Anatolia as a Center of Diversity
7.6. Conclusions and Future Research
Acknowledgements
References
- Brown, JH; Lomolino, MV. Biogeography, 2nd ed; Sinauer Associates: Sunderland, MA, USA, 1998; p. 691. [Google Scholar]
- Telfer, PT; Souquiere, S; Clifford, SL; Abernethy, KA; Bruford, MW; Disotell, TR; Sterner, KN; Roques, P; Marx, PA; Wickings, EJ. Molecular evidence for deep phylogenetic divergence in Mandrillus sphinx. Mol. Ecol 2003, 12, 2019–2024. [Google Scholar]
- Badgley, C; Fox, DL. Ecological biogeography of North American mammals: Species density and ecological structure in relation to environmental gradients. J. Biogeogr 2000, 27, 1437–1467. [Google Scholar]
- Taberlet, P; Fumagalli, L; Wust-Saucy, AG; Cosson, JF. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol 1998, 7, 453–464. [Google Scholar]
- Hewitt, GM. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. Lond 1999, 68, 87–112. [Google Scholar]
- Hewitt, G. Genetic consequences of climatic oscillations in the Quaternary. Philos. Trans. R. Soc. Lond. B Biol. Sci 2004, 359, 183–195. [Google Scholar]
- Remington, CL. Suture-zones of hybrid interaction between recently joined biotas. Evol. Biol 1968, 2, 321–428. [Google Scholar]
- Swenson, NG; Howard, DJ. Clustering of contact zones, hybrid zones, and phylogeographic breaks in North America. Am. Nat 2005, 166, 581–591. [Google Scholar]
- Kosswigg, C. Zoogeography of the Near East. Syst Zool 1955, 4. [Google Scholar]
- Davis, PH. Davis, PH, Harper, PC, Hedge, IC, Eds.; Distribution patterns in Anatolia with particular reference to endemism. In Plant Life of South-West Asia; Edinburgh, Botanical Society of Edinburgh: Edinburgh, Germany, 1971; pp. 15–27. [Google Scholar]
- Demirsoy, A. Genel ve Türkiye Zoocoğrafyası; Meteksan AŞ: Ankara, Turkey, 1996. [Google Scholar]
- Mittermeier, RA; Gil, PR; Hoffman, M; Pilgrim, J; Brooks, T; Mittermeier, JC; Lamoreux, J; da Fonseca, GAB. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; Amsterdam University Press: Amsterdam, The Netherlands, 2005; p. 392. [Google Scholar]
- Koch, MA; Kiefer, C; Ehrich, D; Vogel, J; Brochmann, C; Mummenhoff, K. Three times out of Asia Minor: The phylogeography of Arabis alpina L. (Brassicaceae). Mol. Ecol 2006, 15, 825–839. [Google Scholar]
- Rossiter, SJ; Benda, P; Dietz, C; Zhang, S; Jones, G. Rangewide phylogeography in the greater horseshoe bat inferred from microsatellites: Implications for population history, taxonomy and conservation. Mol. Ecol 2007, 16, 4699–4714. [Google Scholar]
- Rokas, A; Atkinson, RJ; Webster, L; Csoka, G; Stone, GN. Out of Anatolia: Longitudinal gradients in genetic diversity support an eastern origin for a circum-Mediterranean oak gallwasp Andricus quercustozae. Mol. Ecol 2003, 12, 2153–2174. [Google Scholar]
- Veith, M; Schmidtler, JF; Kosuch, J; Baran, I; Seitz, A. Palaeoclimatic changes explain Anatolian mountain frog evolution: A test for alternating vicariance and dispersal events. Mol. Ecol 2003, 12, 185–199. [Google Scholar]
- Bernatchez, L. The evolutionary history of brown trout (Salmo trutta L.) inferred from phylogeographic, nested clade, and mismatch analyses of mitochondrial DNA variation. Evolution 2001, 55, 351–379. [Google Scholar]
- Bilgin, R; Andrzej, F; Çoraman, E; Karataş, A. Phylogeography of the Mediterranean horseshoe bat, Rhinolophus euryale (Chiroptera: Rhinolophidae), in southeastern Europe and Anatolia. Acta Chiropt 2008, 10, 41–49. [Google Scholar]
- Gündüz, I; Rambau, RV; Tez, C; Searle, JB. Mitochondrial DNA variation in the western house mouse (Mus musculus domesticus) close to its site of origin: Studies in Turkey. Biol. J. Linn. Soc 2005, 84, 473–485. [Google Scholar]
- Rajabi-Maham, H; Orth, A; Bonhomme, F. Phylogeography and postglacial expansion of Mus musculus domesticus inferred from mitochondrial DNA coalescent, from Iran to Europe. Mol. Ecol 2008, 17, 627–641. [Google Scholar]
- Ozkan, H; Brandolini, A; Schafer-Pregl, R; Salamini, F. AFLP analysis of a collection of tetraploid wheats indicates the origin of emmer and hard wheat domestication in southeast Turkey. Mol. Biol. Evol 2002, 19, 1797–1801. [Google Scholar]
- Gargani, M; Pariset, L; Soysal, MI; Ozkan, E; Valentini, A. Genetic variation and relationships among Turkish water buffalo populations. Anim. Genet 2010, 41, 93–96. [Google Scholar]
- Kandemir, I; Kence, M; Kence, A. Morphometric and electrophoretic variation in different honeybee (Apis mellifera L.) populations. Turk. J. Vet. Anim. Sci 2005, 29, 885–890. [Google Scholar]
- Kornilios, P; Kyriazi, P; Poulakakis, N; Kumlutas, Y; Ilgaz, C; Mylonas, M; Lymberakis, P. Phylogeography of the ocellated skink Chalcides ocellatus (Squamata, Scincidae), with the use of mtDNA sequences: A Hitch-Hiker‘s guide to the mediterranean. Mol. Phylogenet. Evol 2010, 54, 445–456. [Google Scholar]
- Akın, Ç; Bilgin, C; Beerli, P; Westaway, R; Ohst, T; Litvinchuk, SN; Uzzell, T; Bilgin, M; Hotz, H; Guex, GD. Phylogeographic patterns of genetic diversity in eastern Mediterranean water frogs were determined by geological processes and climate change in the late Cenozoic. J. Biogeogr 2010, 37, 2111–2124. [Google Scholar]
- Gvozdik, V; Moravec, J; Klütsch, C; Kotlik, P. Phylogeography of the Middle Eastern tree frogs (Hyla, Hylidae, Amphibia) as inferred from nuclear and mitochondrial DNA variation, with a description of a new species. Mol. Phylogen. Evol 2010, 55, 1146–1166. [Google Scholar]
- Poulakakis, N; Pakaki, V; Mylonas, M; Lymberakis, P. Molecular phylogeny of the Greek legless skink Ophiomorus punctatissimus (Squamata: Scincidae): The impact of the mid-Aegean trench in its phylogeography. Mol. Phylogenet. Evol 2008, 47, 396–402. [Google Scholar]
- Kotlik, P; Markova, S; Choleva, L; Bogutskaya, NG; Ekmekci, FG; Ivanova, PP. Divergence with gene flow between Ponto-Caspian refugia in an anadromous cyprinid Rutilus frisii revealed by multiple gene phylogeography. Mol. Ecol 2008, 17, 1076–1088. [Google Scholar]
- Castiglia, R; Annesi, F; Krystufek, B; Filippucci, M; Amori, G. The evolutionary history of a mammal species with a highly fragmented range: The phylogeography of the European snow vole. J Zool 2009, 279, 243–250. [Google Scholar]
- Juste, J; Ibanez, C; Munoz, J; Trujillo, D; Benda, P; Karatas, A; Ruedi, M. Mitochondrial phylogeography of the long-eared bats (Plecotus) in the Mediterranean Palaearctic and Atlantic islands. Mol. Phylogenet. Evol 2004, 31, 1114–1126. [Google Scholar]
- Pasmans, F; Bogaerts, S; Woeltjes, T; Carranza, S. Biogeography of Neurergus strauchii barani Öz, 1994 and N. s. strauchii (Steindachner, 1887) (Amphibia: Salamandridae) assessed using morphological and molecular data. Amphibia-Reptilia 2006, 27, 281–288. [Google Scholar]
- Hrbek, T; Meyer, A. Closing of the Tethys Sea and the phylogeny of Eurasian killifishes (Cyprinodontiformes: Cyprinodontidae). J. Evol. Biol 2003, 16, 17–36. [Google Scholar]
- Triantafyllidis, A; Leonardos, I; Bista, I; Kyriazis, ID; Stoumboudi, MT; Kappas, I; Amat, F; Abatzopoulos, TJ. Phylogeography and genetic structure of the Mediterranean killifish Aphanius fasciatus (Cyprinodontidae). Mar. Biol 2007, 152, 1159–1167. [Google Scholar]
- Bittkau, C; Comes, HP. Evolutionary processes in a continental island system: Molecular phylogeography of the Aegean Nigella arvensis alliance (Ranunculaceae) inferred from chloroplast DNA. Mol. Ecol 2005, 14, 4065–4083. [Google Scholar]
- Bilgin, R; Karataş, A; Çoraman, E; Morales, JC. The mitochondrial and nuclear genetic structure of Myotis capaccinii (Chiroptera: Vespertilionidae) in the Eurasian transition, and its taxonomic implications. Zool. Scr 2008, 37, 253–262. [Google Scholar]
- Stöck, M; Moritz, C; Hickerson, M; Frynta, D; Dujsebayeva, T; Eremchenko, V; Macey, JR; Papenfuss, TJ; Wake, DB. Evolution of mitochondrial relationships and biogeography of Palearctic green toads (Bufo viridis subgroup) with insights in their genomic plasticity. Mol. Phylogenet. Evol 2006, 41, 663–689. [Google Scholar]
- Poulakakis, N; Lymberakis, P; Tsigenopoulos, CS; Magoulas, A; Mylonas, M. Phylogenetic relationships and evolutionary history of snake-eyed skink Ablepharus kitaibelii (Sauria: Scincidae). Mol. Phylogenet. Evol 2005, 34, 245–256. [Google Scholar]
- Kasapidis, P; Suchentrunk, F; Magoulas, A; Kotoulas, G. The shaping of mitochondrial DNA phylogeographic patterns of the brown hare (Lepus europaeus) under the combined influence of late Pleistocene climatic fluctuations and anthropogenic translocations. Mol. Phylogenet. Evol 2005, 34, 55–66. [Google Scholar]
- Stamatis, C; Suchentrunk, F; Moutou, KA; Giacometti, M; Haerer, G; Djan, M; Vapa, L; Vukovic, M; Tvrtkovic, N; Sert, H; Alves, PC; Mamuris, Z. Phylogeography of the brown hare (Lepus europaeus) in Europe: A legacy of south-eastern Mediterranean refugia? J. Biogeogr 2009, 36, 515–528. [Google Scholar]
- King, RA; Ferris, C. Chloroplast DNA phylogeography of Alnus glutinosa (L.) Gaertn. Mol. Ecol 1998, 7, 1151–1161. [Google Scholar]
- Hampe, A; Arroyo, J; Jordano, P; Petit, RJ. Rangewide phylogeography of a bird-dispersed Eurasian shrub: Contrasting Mediterranean and temperate glacial refugia. Mol. Ecol 2004, 12, 3415–3426. [Google Scholar]
- Michaux, J; Libois, R; Paradis, E; Filippucci, M. Phylogeographic history of the yellow-necked fieldmouse (Apodemus flavicollis) in Europe and in the Near and Middle East. Mol. Phylogen. Evol 2004, 32, 788–798. [Google Scholar]
- Dubey, S; Cosson, J; Vohralik, V; Krystufek, B; Diker, E; Vogel, P. Molecular evidence of Pleistocene bidirectional faunal exchange between Europe and the Near East: The case of the bicoloured shrew (Crocidura leucodon, Soricidae). J. Evol. Biol 2007, 20, 1799–1808. [Google Scholar]
- Cooper, SJ; Ibrahim, KM; Hewitt, GM. Postglacial expansion and genome subdivision in the European grasshopper Chorthippus parallelus. Mol. Ecol 1995, 4, 49–60. [Google Scholar]
- Gündüz, I; Jaarola, M; Tez, C; Yeniyurt, C; Polly, PD; Searle, JB. Multigenic and morphometric differentiation of ground squirrels (Spermophilus, Scuiridae, Rodentia) in Turkey, with a description of a new species. Mol. Phylogenet. Evol 2007, 43, 916–935. [Google Scholar]
- Bilgin, R; Çoraman, E; Karataş, A; Morales, JC. Phylogeography of the greater horseshoe bat, Rhinolophus ferrumequinum (Chiroptera: Rhinolophidae), in southeastern Europe and Anatolia, with a specific focus on whether the Sea of Marmara is a barrier to gene flow. Acta Chiropterol 2009, 11, 53–60. [Google Scholar]
- Rossiter, SJ; Benda, P; Dietz, C; Zhang, S; Jones, G. Rangewide phylogeography in the greater horseshoe bat inferred from microsatellites: Implications for population history, taxonomy and conservation. Mol. Ecol 2007, 16, 4699–4714. [Google Scholar]
- Bilgin, R; Karataş, A; Çoraman, E; Pandurski, I; Papadatou, E; Morales, JC. Molecular taxonomy and phylogeography of Miniopterus schreibersii (Kuhl, 1817) (Chiroptera: vespertilionidae), in the Eurasian transition. Biol. J. Linn. Soc 2006, 87, 577–582. [Google Scholar]
- Furman, A; Çoraman, E; Bilgin, R; Karataş, A. Molecular ecology and phylogeography of the bent-wing bat complex (Miniopterus schreibersii) (Chiroptera: Vespertilionidae) in Asia Minor and adjacent regions. Zool. Scr 2009, 38, 129–141. [Google Scholar]
- Bilgin, R; Karatas, A; Coraman, E; Disotell, T; Morales, JC. Regionally and climatically restricted patterns of distribution of genetic diversity in a migratory bat species, Miniopterus schreibersii (Chiroptera: Vespertilionidae). BMC Evol Biol 2008, 8. [Google Scholar] [CrossRef]
- Furman, A; Postawa, T; Oztunc, T; Coraman, E. Cryptic diversity of the bent-wing bat, Miniopterus schreibersii (Chiroptera: Vespertilionidae), in Asia Minor. BMC Evol Biol 2010, 10. [Google Scholar] [CrossRef]
- Wielstra, B; Espregueira Themudo, G; Güçlü, Ö; Olgun, K; Poyarkov, N; Arntzen, J. Cryptic crested newt diversity at the Eurasian transition: The mitochondrial DNA phylogeography of Near Eastern Triturus newts. Mol. Phylogen. Evol 2010, 56, 888–896. [Google Scholar]
- Wahlberg, N; Saccheri, I. The effects of Pleistocene glaciations on the phylogeography of Melitaea cinxia (Lepidoptera: Nymphalidae). Eur. J. Entomol 2007, 104, 675–684. [Google Scholar]
- Jakob, SS; Ihlow, A; Blattner, FR. Combined ecological niche modelling and molecular phylogeography revealed the evolutionary history of Hordeum marinum (Poaceae)—niche differentiation, loss of genetic diversity and speciation in Mediterranean Quaternary refugia. Mol. Ecol 2007, 16, 1713–1727. [Google Scholar]
- Simonato, M; Mendel, Z; Kerdelhue, C; Rousselet, J; Magnoux, E; Salvato, P; Roques, A; Battisti, A; Zane, L. Phylogeography of the pine processionary moth Thaumetopoea wilkinsoni in the Near East. Mol. Ecol 2007, 16, 2273–2284. [Google Scholar]
- Dubey, S; Zaitsev, M; Cosson, JF; Abdukadier, A; Vogel, P. Pliocene and Pleistocene diversification and multiple refugia in a Eurasian shrew (Crocidura suaveolens group). Mol. Phylogenet. Evol 2006, 38, 635–647. [Google Scholar]
- Dubey, S; Cosson, J. Mediterranean populations of the lesser white toothed shrew (Crocidura suaveolens group): An unexpected puzzle of Pleistocene survivors and prehistoric introductions. Mol. Ecol 2007, 16, 3438–3452. [Google Scholar]
- Dubey, S; Diker, E; Kurtonur, C; Vogel, P. Secondary contact zones and hybridizations: The case of the lesser white toothed shrew (Crocidura suaveolens group, Soricidae). Biol. J. Linn. Soc 2008, 95, 557–565. [Google Scholar]
- Stöck, M; Dubey, S; Klütsch, C; Litvinchuk, L; Scheidt, U; Perrin, N. Mitochondrial and nuclear phylogeny of circum-Mediterranean tree frogs from the Hyla arborea group. Mol. Phylogenet. Evol 2008, 49, 1019–1024. [Google Scholar]
- Filippucci, MG; Simson, S. Allozyme variation and divergence in Erinaceidae (Mammalia: Insectivora). Isr. J. Zool 1996, 42, 335–345. [Google Scholar]
- Santucci, F; Emerson, BC; Hewitt, GM. Mitochondrial DNA phylogeography of European hedgehogs. Mol. Ecol 1998, 7, 1163–1172. [Google Scholar]
- Seddon, JM; Santucci, F; Reeve, N; Hewitt, GM. Caucasus mountains divide postulated postglacial colonization routes in the white-breasted hedgehog, Erinaceus concolor. J. Evol. Biol 2002, 15, 463–467. [Google Scholar]
- Çıplak, B; Gündüz, I; Kaya, S. Phylogeography of Anterastes serbicus species group (Orthoptera, Tettigoniidae): Phylogroups correlate with mountain belts, but not with the morphospecies. J. Orthoptera Res 2010, 19, 89–100. [Google Scholar]
- Bardakci, F; Degerli, N; Ozdemir, O; Basibuyuk, HH. Phylogeography of the Turkish brown trout Salmo trutta L.: Mitochondrial DNA PCR-RFLP variation. J. Fish. Biol 2006, 68, 36–55. [Google Scholar]
- Durand, JD; Unlu, E; Doadrio, I; Pipoyan, S; Templeton, AR. Origin, radiation, dispersion and allopatric hybridization in the chub Leuciscus cephalus. Proc. R. Soc. Biol. Sci. Ser. B 2000, 267, 1687–1697. [Google Scholar]
- Heuertz, M; Carnevale, S; Fineschi, S; Sebastiani, F; Hausman, J; Paule, L; Vendramin, G. Chloroplast DNA phylogeography of European ashes, Fraxinus sp. (Oleaceae): Roles of hybridization and life history traits. Mol. Ecol 2006, 15, 2131–2140. [Google Scholar]
- Strid, A. Phytogeographia Aegaea and the flora Hellenica database. Ann. Nathist. Mus. Wien 1996, 98, 279–289. [Google Scholar]
- Korkmaz, EM; Sarı, M; Başıbüyük, HH. Genetic structure of Chorthippus parallelus (Orthoptera: Acrididae: Gomphocerinae) populations in Anatolia: A stable rear edge population. Ann. Entomol. Soc. Am 2010, 103, 625–634. [Google Scholar]
- Atkinson, RJ; Rokas, A; Stone, GN. Weiss, S, Ferrand, N, Eds.; Phylogeography of Southern European Refugia: Evolutionary Perspectives on the Origins and Conservation of European Biodiversity; Springer: Dordrecht, The Netherlands, 2007; p. 377. [Google Scholar]
- Winograd, IJ; Landwehr, JM; Ludwig, KR; Coplen, TB; Riggs, AC. Duration and structure of the past four interglaciations. Quat. Res 1997, 48, 141–154. [Google Scholar]
- Hewitt, G. Hybrid zones-natural laboratories for evolutionary studies. Trends Ecol. Evol 1988, 3, 158–167. [Google Scholar]
- Erinç, S. Brice, WC, Ed.; Changes in the physical environment in Turkey since the end of the last glacial. In The Environmental History of the Near and Middle East Since the Last Ice Age; Academic Press: London, UK, 1978. [Google Scholar]
- Ibrahim, K; Nichols, RA; Hewitt, GM. Spatial patterns of genetic variation generated by different forms of dispersal during range expansion. Heredity 1996, 77, 282–291. [Google Scholar]
- Mayr, E; Ashlock, PD. Principles of Systematic Zoology, 2nd ed; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Çıplak, B. Fattorini, S, Ed.; The analogy between interglacial and global warming for the glacial relicts in a refugium: A biogeographic perspective for conservation of Anatolian Orthoptera. In Insect Ecology and Conservation; Research Signpost Inc: Kerela, India, 2008; pp. 135–163. [Google Scholar]
- Çıplak, B; Demirsoy, A; Bozcuk, AN. Distribution of Orthoptera in relation to the Anatolian Diagonal in Turkey. Articulata 1993, 8, 1–20. [Google Scholar]
- Steininger, FF; Rabeder, G; Rögl, FL. Stanly, DJ, Wezel, FC, Eds.; Geological Evolution of the Mediterranean Basin; Springer: New York, NY, USA, 1985; pp. 559–571. [Google Scholar]
- Yesson, C; Culham, A. A phyloclimatic study of Cyclamen. BMC Evol Biol 2006, 6. [Google Scholar] [CrossRef]
- Gomez, A; Lunt, DH. Weiss, S, Ferrand, N, Eds.; Refugia within refugia: Patterns of phylogeographic concordance in the Iberian peninsula. In Phylogeography of Southern European Refugia; Springer: Dordrecht, The Netherlands, 2007; p. 377. [Google Scholar]
- Garcia-Mudarra, JL; Ibanez, C; Juste, J. The Straits of Gibraltar: Barrier or bridge to Ibero-Moroccan bat diversity? Biol. J. Linn. Soc 2009, 96, 434–450. [Google Scholar]
- Medail, F; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean basin. J. Biogeogr 2009, 36, 1333–1345. [Google Scholar]




| Species | Marker | Age of Divergence | Pattern | Reference |
|---|---|---|---|---|
| Killifish, Aphanius fasciatus | RFLPs and mtDNA sequencing | Pliocene (4 Mya) | I | [32,33] |
| Love-in-a-mist, Nigella arvensis alliance | PCR-RFLP | Pleistocene (<1 Mya) | I | [34] |
| Long fingered bat, Myotis capaccinnii | mtDNA sequencing and microsatellites | Pleistocene (500 Kya) | I | [35] |
| The European green toad, Bufo viridis (2n) | mtDNA sequencing | Pliocene (4.8–3.6 Mya) | I | [36] |
| Snake-eyed skink, Ablepharus kitaibelii | mtDNA sequencing | Pliocene (5.9–5.7 Mya) | I | [37] |
| Brown Hare, Lepus europaeus | RFLPs and mtDNA seqeuncing | Pleistocene (490–105 Kya) | I* | [38,39] |
| Black Alder, Alnus glutinosa | RFLPs | N/A | I | [40] |
| Eurasian shrub, Frangula alnus | RFLPs | N/A | I | [41] |
| Yellow-necked fieldmouse, Apodemus flavicollis | mtDNA sequencing | Pliocene (2.4–2.2 Mya) | I | [42] |
| Bicolored shrew, Crocidura leucodon | mtDNA and nuclear sequencing | 0.691 Mya (CI: 0.510–0.980) | I | [43] |
| European grasshopper, Chorthippus parallelus | nuclear sequencing | N/A | I | [44] |
| Ground squirrels, Spermophilus spp | mtDNA, sequencing (X and Y chrom.) | N/A | II | [45] |
| Mountain frog, Rana macrocnemis | mtDNA sequencing | Pliocene (2.4 Mya) | II | [16] |
| Greater horseshoe bat, Rhinolophus ferrumequinum | mtDNA sequencing and microsatellites | Pleistocene (350–750 Kya) | II | [46,47] |
| Bent-winged bat, Miniopterus schreibersii | mtDNA sequencing and microsatellites | Pleistocene (170–300 Kya) | II | [48,49,50, 51] |
| The crested newt, Triturus karelinii | mtDNA sequencing | Pliocene (5.5 Mya) | II | [52] |
| Glanville fritillary, Melitaea cinxia | mtDNA sequencing | N/A | II | [53] |
| Annual grass, Hordeum gussoneanum | Chloroplast sequencing, microsatellites | N/A | II* | [54] |
| Pine processionary moth, Thaumetopoea wilkinsoni | mtDNA sequencing, AFLPs, microsats | Pleistocene (1.5–0.5 Mya) | II | [55] |
| Lesser white-toothed shrew, Crocidura suaveolens | mtDNA and nuclear gene sequencing | Pleistocene (940 Kya) | I&II | [56,57,58] |
| Tree frog, Hyla arborea | mtDNA and nDNA sequencing | N/A | I&II | [59] |
| White-breasted hedgehog, Erinaceous concolor | Allozymes and mtDNA sequencing | Pliocene 3 Mya (B-A) | I & II | [60,61,62] |
| Anterastes serbicus group | mtDNA sequencing | Plesitocene, <1.56 Mya | I&II | [63] |
| Oak-gallwasp, Andricus quercutozae | mtDNA sequencing and allozymes | Pliocene (7 Mya) | I&II* | [15] |
| Brown trout, Salmo trutta | RFLPs | Late Pleistocene | I&II* | [17,64] |
| Chub, Leuciscus cephalus | mtDNA sequencing | Pliocene 3–2.5 Mya | I&II* | [65] |
| Alpine rockcress, Arabis alpina | C.plast and nDNA sequencing | N/A | * | [13] |
| European ash, Fraxinus angustifolia | Chloroplast microsatellites | N/A | * | [66] |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bilgin, R. Back to the Suture: The Distribution of Intraspecific Genetic Diversity in and Around Anatolia. Int. J. Mol. Sci. 2011, 12, 4080-4103. https://doi.org/10.3390/ijms12064080
Bilgin R. Back to the Suture: The Distribution of Intraspecific Genetic Diversity in and Around Anatolia. International Journal of Molecular Sciences. 2011; 12(6):4080-4103. https://doi.org/10.3390/ijms12064080
Chicago/Turabian StyleBilgin, Rasit. 2011. "Back to the Suture: The Distribution of Intraspecific Genetic Diversity in and Around Anatolia" International Journal of Molecular Sciences 12, no. 6: 4080-4103. https://doi.org/10.3390/ijms12064080




