Selective Growth of α-Sexithiophene by Using Silicon Oxides Patterns
Abstract
:1. Introduction
2. Experimental and Methods
2.1. Fabrication of SiOx Patterned Surfaces
2.2. Organic Molecular Beam Deposition of 6T Ultra-Thin Films
2.3. Atomic Force Microscopy Measurements
2.4. Image Processing of 6T Islands: Angular Orientation, Roughness, Relative Position and Surface Coverage
2.5. In-Situ and Real-Time X-Ray Measurements
3. Results and Discussion
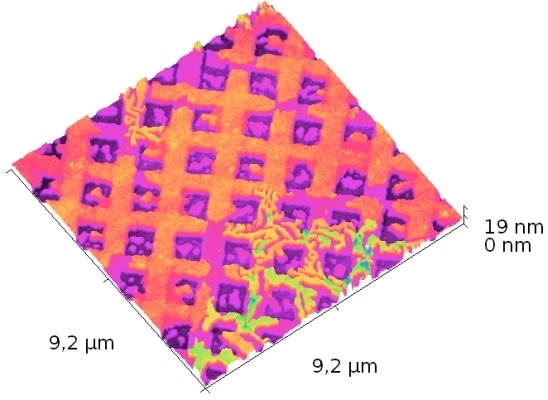
3.1. Pattern vs. Film Thickness: from Sub-Monolayer to Bulk
3.2. Thermal Annealing and Thermal Stability of 6T Films
3.3. Enhancement of the 6T Selective Growth by Means of Electric Field and Grid Pattern
4. Conclusions
Acknowledgments
References and Notes
- Koh, SJ. Strategies for controlled placement of nanoscale building blocks. Nanoscale Res Lett 2007, 2, 519–545. [Google Scholar]
- Song, H; Reed, MA; Lee, T. Single-molecule devices: Single molecule electronic devices. Adv Mater 2011, 23, 1583–1608. [Google Scholar]
- Khajetoorians, AA; Wiebe, J; Chilian, B; Wiesendanger, R. Realizing all-spin–based logic operations atom by atom. Science 2011, 332, 1062–1064. [Google Scholar]
- Shehu, A; Quiroga, SD; D’Angelo, P; Albonetti, C; Borgatti, F; Murgia, M; Scorzoni, A; Stoliar, P; Biscarini, F. Layered distribution of charge carriers in organic thin film transistors. Phys Rev Lett 2010, 104, 246602:1–246602:4. [Google Scholar]
- Cavallini, M; Albonetti, C; Biscarini, F. Nanopatterning soluble multifunctional materials by unconventional wet lithography. Adv Mater 2009, 21, 1043–1053. [Google Scholar]
- Melucci, M; Zambianchi, M; Favaretto, L; Palermo, V; Treossi, E; Montalti, M; Bonacchi, S; Cavallini, M. Multicolor, large-area fluorescence sensing through oligothiophene-self-assembled monolayers. Chem Commun 2011, 47, 1689–1691. [Google Scholar]
- Melucci, M; Favaretto, L; Zanelli, A; Cavallini, M; Bongini, A; Maccagnani, P; Ostoja, P; Derue, G; Lazzaroni, R; Barbarello, G. Thiophene-benzothiadiazole co-oligomers: Synthesis, optoelectronic properties, electrical characterization, and thin-film patterning. Adv Funct Mater 2010, 20, 445–452. [Google Scholar]
- Sirringhaus, H; Shimoda, T. Inkjet printing of functional materials. MRS Bull 2003, 28, 802–803. [Google Scholar]
- Dinelli, F; Murgia, M; Levy, P; Cavallini, M; Biscarini, F; de Leeuw, DM. Spatially correlated charge transport in organic thin film transistors. Phys Rev Lett 2004, 92, 116802:1–116802:4. [Google Scholar]
- Dediu, VA; Hueso, LE; Bergenti, I; Taliani, C. Spin routes in organic semiconductors. Nat Mater 2009, 8, 707–716. [Google Scholar]
- Capelli, R; Toffanin, S; Generali, G; Usta, H; Facchetti, A; Muccini, M. Organic light-emitting transistors with an efficiency that outperforms the equivalent light-emitting diodes. Nat Mater 2010, 9, 496–503. [Google Scholar]
- Garcia, R; Tello, M; Moulin, JF; Biscarini, F. Size and shape controlled growth of molecular nanostructures on silicon oxide templates. Nano Lett 2004, 4, 1115–1119. [Google Scholar]
- Losilla, NS; Martinez, J; Bystrenova, E; Greco, P; Biscarini, F; García, R. Patterning pentacene surfaces by local oxidation nanolithography. Ultramicroscopy 2010, 110, 729–732. [Google Scholar]
- Steudel, S; Janssen, D; Verlaak, S; Genoe, J; Heremans, P. Patterned growth of pentacene. Appl Phys Lett 2004, 85, 5550–5552. [Google Scholar]
- Brinkmann, M; Graff, S; Biscarini, F. Mechanism of nonrandom pattern formation of polar-conjugated molecules in a partial wetting regime. Phys Rev B 2002, 66, 165430:1–165430:8. [Google Scholar]
- Albonetti, C; Martinez, J; Losilla, NS; Greco, P; Cavallini, M; Borgatti, F; Montecchi, M; Pasquali, L; Garcia, R; Biscarini, F. Parallel-local anodic oxidation of silicon surfaces by soft stamps. Nanotechnology 2008, 19, 435303. [Google Scholar] [CrossRef]
- Cavallini, M; Mei, P; Biscarini, F; García, R. Parallel writing by local oxidation nanolithography with submicrometer resolution. Appl Phys Lett 2003, 83, 5286–5288. [Google Scholar]
- Zhao, X-M; Xia, YN; Whitesides, GM. Soft lithographic methods for nano-fabrication. J Mater Chem 1997, 7, 1069–1074. [Google Scholar]
- Dinelli, F; Moulin, J-F; Loi, MA; da Como, E; Massi, M; Murgia, M; Muccini, M; Biscarini, F; Wie, J; Kingshott, P. Effects of surface chemical composition on the early growth stages of alpha-sexithienyl films on silicon oxide substrates. J Phys Chem B 2006, 110, 258–263. [Google Scholar]
- We define a monolayer (ML) as a layer of ordered and closed packed molecules orthogonal to the surface that completely covers it (Θ = 100%). A nominal ML is equivalent to 24 Å of 6T molecules deposited on the SiOx substrate.
- Viville, BP; Lazzaroni, R; Bredas, JL; Moretti, P; Samorí, P; Biscarini, F. The influence of thermal annealing on the morphology of sexithienyl thin films. Adv Mater 1998, 57–60. [Google Scholar]
- García, R; Perez, R. Dynamic atomic force microscopy methods. Surf Sci Rep 2002, 47, 197–301. [Google Scholar]
- Alexeev, A. Study of monomolecular layers of azobenzene derivative by the scanning probe microscopy. Mater Sci Eng C 2002, 22, 453–458. [Google Scholar]
- Wu, Y; Toccoli, T; Koch, N; Iacob, E; Pallaoro, A; Rudolf, P; Iannotta, S. Controlling the early stages of pentacene growth by supersonic molecular beam deposition. Phys Rev Lett 2007, 98, 076601:1–076601:4. [Google Scholar]
- Dinelli, F; Albonetti, C; Kolosov, OV. Ultrasonic force microscopy: Detection and imaging of ultra-thin molecular domains. Ultramicroscopy 2011, 111, 267–272. [Google Scholar]
- Necas, D; Klapetek, P. Gwyddion Free Software. Available online: http://gwyddion.net/ (accessed on 31 August 2011).
- Klapetek, P; Necas, D; Anderson, C. Gwyddion User Guide, version 2009-11-11. Available online: http://gwyddion.net (accessed on 5 September 2011).
- Straley, JP. Ordered phases of a liquid of biaxial particles. Phys Rev 1974, 10, 1881–1887. [Google Scholar]
- Rasband, WS. Image J. Available online: http://rsbweb.nih.gov/ij/ (accessed on 31 August 2011).
- Moulin, J-F; Dinelli, F; Massi, M; Albonetti, C; Kshirsagar, R; Biscarini, F. In situ X-ray synchrotron study of organic semiconductor ultra-thin films growth. Nucl Instrum Methods Phys Res Sect B 2006, 246, 122–126. [Google Scholar]
- Chua, L-L; Dipankar, M; Sivaramakrishnan, S; Gao, X; Qi, D; Wee, ATS; Ho, PKH. Large damage threshold and small electron escape depth in X-ray absorption spectroscopy of a conjugated polymer thin film. Langmuir 2006, 22, 8587–8594. [Google Scholar]
- Pratontep, S; Brinkmann, M; Nüesch, F; Zuppiroli, L. Correlated growth in ultrathin pentacene films on silicon oxide: Effect of deposition rate. Phys Rev B 2004, 69, 165201. [Google Scholar] [CrossRef]
- Martinez, NF; Patil, S; Lozano, JR; Garcia, R. Enhanced compositional sensitivity in atomic force microscopy by the excitation of the first two flexural modes. Appl Phys Lett 2006, 89, 153115. [Google Scholar] [CrossRef]
- Martínez, NF; Kamiński, W; Gómez, CJ; Albonetti, C; Biscarini, F; Pérez, R; García, R. Molecular scale energy dissipation in oligothiophene monolayers measured by dynamic force microscopy. Nanotechnology 2009, 20, 434021. [Google Scholar] [CrossRef]
- The surface coverage Θ is the sum of the islands areas projected on the SiOx plane therefore an oblique angle decrease the projected area (cf. 2.4).
- Standardized one-dimensional Rq is evaluated from line profiles (10 μm long and 15 lines thick) taken along both top and basal surfaces.
- Besana, D; Borghesi, A; Campione, M; Sassella, A; Tubino, R; Moret, M; Rinaldi, R; Garnier, F. Influence of the substrate on the growth of [alpha],[omega]-dihexyl-quaterthiophene thin films by organic molecular beam deposition. J Cryst Growth 2002, 235, 241–247. [Google Scholar]
- Matsumoto, Y. Novel prevention method of stiction using silicon anodization for SOI structure. Sens Actuators A 1999, 72, 153–159. [Google Scholar]
- Forrest, SR. Ultrathin organic films grown by organic molecular beam deposition and related techniques. Chem Rev 1997, 97, 1793–1896. [Google Scholar]
- Horowitz, G; Bachet, B; Yassar, A; Lang, P; Demanze, F; Fave, JL; Garnier, F. Growth and characterization of sexithiophene single-crystals. Chem Mater 1995, 7, 1337–1341. [Google Scholar]
- Sherrer, P. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen; Göttinger Digitalisierungszentrum: Goettingen, Germany, 1918; pp. 98–100. [Google Scholar]
- Milita, S; Dionigi, C; Borgatti, F; Lazar, AN; Porzio, W; Destri, S; Wermeille, D; Felici, R; Andreasen, JW; Nielsen, MM; et al. Crystallization of organic semiconductor molecules in nanosized cavities: Mechanism of polymorphs formation studied by in situ XRD. J Phys Chem C 2008, 112, 12177–12183. [Google Scholar]
- Ikeda, S; Saiki, K; Wada, Y; Inaba, K; Ito, Y; Kikuchi, H; Terashima, K; Shimada, T. Graphoepitaxy of sexithiophene and orientation control by surface treatment. J Appl Phys 2008, 103, 084313:1–084313:9. [Google Scholar]
- Biscarini, F; Samorí, P; Greco, O; Zamboni, R. Scaling behavior of anisotropic organic thin films grown in high vacuum. Phys Rev Lett 1997, 78, 2389–2392. [Google Scholar]
- Muccini, M; Murgia, M; Biscarini, F. Morphology controlled energy transfer in conjugated molecular thin films. Adv Mater 2001, 13, 355–358. [Google Scholar]
- Biscarini, F; Zamboni, R; Samorì, P; Ostoja, P; Taliani, C. Growth of conjugated oligomer thin films studied by atomic-force microscopy. Phys Rev B 1995, 52, 14868–14877. [Google Scholar]
- Taliani, C; Zamboni, R; Ruani, G; Rossini, S; Lazzaroni, R. New rigid rod liquid-crystal molecule precursor of conjugated polymers: Alpha-sexithienyl. J Mol Electron 1990, 6, 225–226. [Google Scholar]
- Pizzirusso, A; Savini, M; Muccioli, L; Zannoni, C. An atomistic simulation of the liquid-crystalline phases of sexithiophene. J Mater Chem 2011, 21, 125–133. [Google Scholar]
- Moulin, J-F; Kengne, JC; Kshirsagar, R; Cavallini, M; Biscarini, F; León, S; Zerbetto, F; Bottari, G; Leigh, DA. Self-organization of rotaxane thin films into spatially correlated nanostructures: Morphological and structural aspects. J Am Chem Soc 2006, 128, 526–532. [Google Scholar]
- Reiter, G. Unstable thin polymer films: Rupture and dewetting processes. Langmuir 1993, 9, 1344–1351. [Google Scholar]
- De Luca, G; Pisula, W; Credgington, D; Treossi, E; Fenwick, O; Lazzerini, GM; Dabirian, R; Orgiu, E; Liscio, A; Palermo, V; et al. Non-conventional processing and post-processing methods for the nanostructuring of conjugated materials for organic electronics. Adv Funct Mater 2011, 21, 1279–1295. [Google Scholar]
- The fractal dimension Df is the scaling exponent of the power law between the perimeter P and the area A of 6T islands
- Meyer zu Heringdorf, FJ; Reuter, MC; Tromp, RM. Growth dynamics of pentacene thin films. Nature 2001, 412, 517–520. [Google Scholar]
- Amar, J. Kinetics of submonolayer epitaxial growth. Comput Phys Commun 2002, 146, 1–8. [Google Scholar]
- Witten, TA; Sander, LM. Diffusion-limited aggregation, a kinetic critical phenomenon. Phys Rev Lett 1981, 47, 1400–1403. [Google Scholar]
- Xie, R; Karim, A; Douglas, J; Han, C; Weiss, R. Spinodal dewetting of thin polymer films. Phys Rev Lett 1998, 81, 1251–1254. [Google Scholar]
- Losilla, NS; Martínez, J; García, R. Large area nanoscale patterning of silicon surfaces by parallel local oxidation. Nanotechnology 2009, 20, 475304. [Google Scholar] [CrossRef]
- Chiesa, M; Garcia, R. Nanoscale space charge generation in local oxidation nanolithography. Appl Phys Lett 2010, 96, 263112. [Google Scholar] [Green Version]
- Losilla, NS; Oxtoby, NS; Martinez, J; Garcia, F; Garcia, R; Mas-Torrent, M; Veciana, J; Rovira, C. Sub-50 nm positioning of organic compounds onto silicon oxide patterns fabricated by local oxidation nanolithography. Nanotechnology 2008, 19, 455308. [Google Scholar] [CrossRef]
- Annibale, P; Albonetti, C; Stoliar, P; Biscarini, F. High-resolution mapping of the electrostatic potential in organic thin-film transistors by phase electrostatic force microscopy. J Phys Chem A 2007, 111, 12854–12858. [Google Scholar]








| η (°) | S | Θstripe (%) | |
|---|---|---|---|
| Ground | 0.81 ± 0.08 | 0.62 ± 0.03 | 73 ± 5 |
| 15 V | 0.6 ± 0.1 | 0.75 ± 0.03 | 86 ± 4 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Albonetti, C.; Barbalinardo, M.; Milita, S.; Cavallini, M.; Liscio, F.; Moulin, J.-F.; Biscarini, F. Selective Growth of α-Sexithiophene by Using Silicon Oxides Patterns. Int. J. Mol. Sci. 2011, 12, 5719-5735. https://doi.org/10.3390/ijms12095719
Albonetti C, Barbalinardo M, Milita S, Cavallini M, Liscio F, Moulin J-F, Biscarini F. Selective Growth of α-Sexithiophene by Using Silicon Oxides Patterns. International Journal of Molecular Sciences. 2011; 12(9):5719-5735. https://doi.org/10.3390/ijms12095719
Chicago/Turabian StyleAlbonetti, Cristiano, Marianna Barbalinardo, Silvia Milita, Massimiliano Cavallini, Fabiola Liscio, Jean-François Moulin, and Fabio Biscarini. 2011. "Selective Growth of α-Sexithiophene by Using Silicon Oxides Patterns" International Journal of Molecular Sciences 12, no. 9: 5719-5735. https://doi.org/10.3390/ijms12095719