Enhancement of Commercial Antifungal Agents by Kojic Acid
Abstract
:1. Introduction
2. Results and Discussion
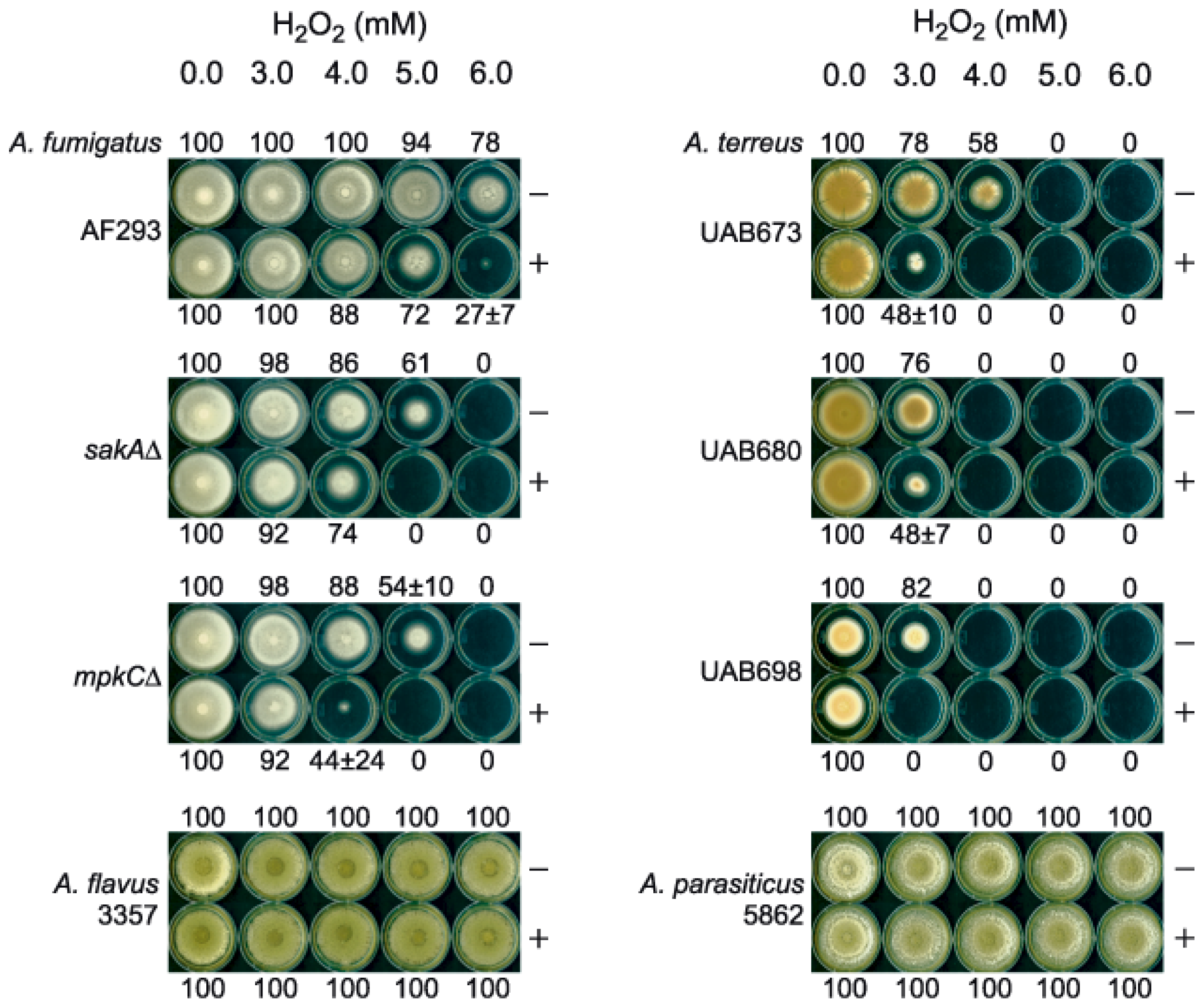
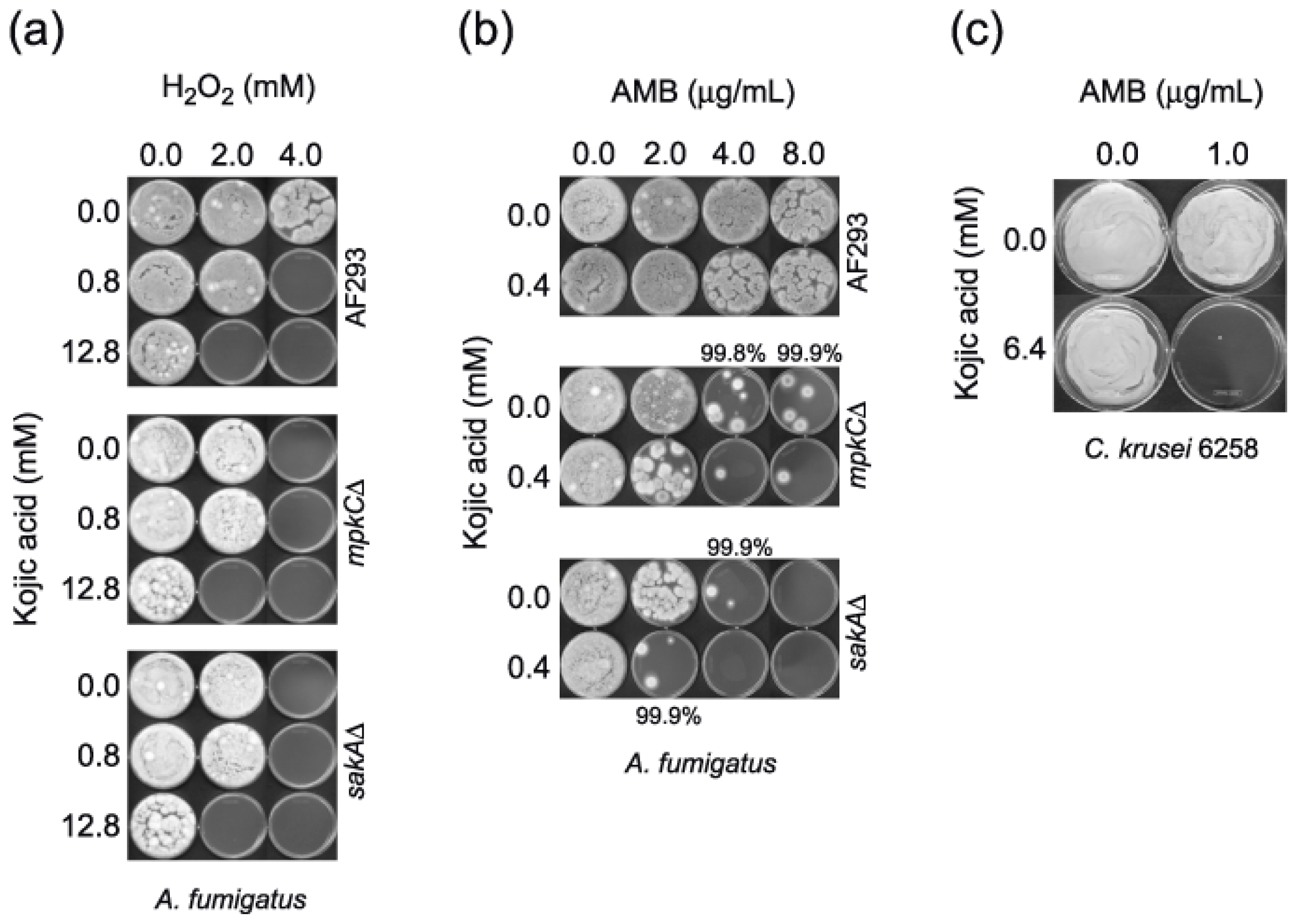
2.1. Enhanced Antimycotic Activity of H2O2 by KA against Filamentous Fungi
2.1.1. Agar Plate Bioassay: Filamentous Fungi
2.1.2. Microtiter Plate (microdilution) Bioassay: Filamentous Fungi
2.2. Enhanced Antimycotic Activity of AMB with KA in Filamentous Fungi and Yeasts
2.2.1. Microtiter Plate (microdilution) Bioassay: Filamentous Fungi
2.2.2. Microtiter Plate (microdilution) Bioassay: Yeasts
2.3. No Enhancement of Antimycotic Activity of H2O2 with KA in Yeasts
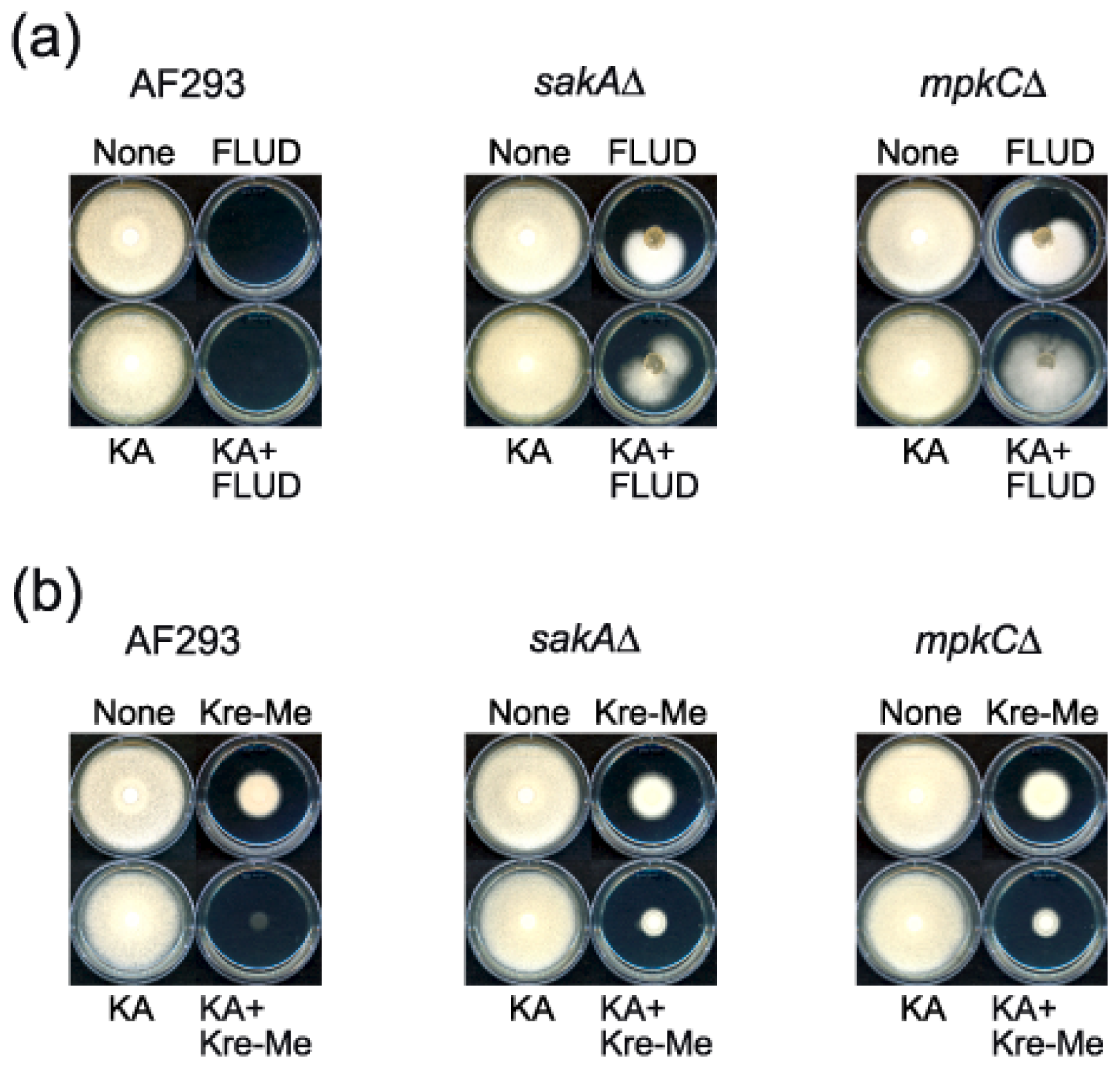
2.4. Enhanced Antimycotic Activity of Strobilurin with KA in A. fumigatus
3. Experimental Section
3.1. Fungal Strains and Culture Conditions
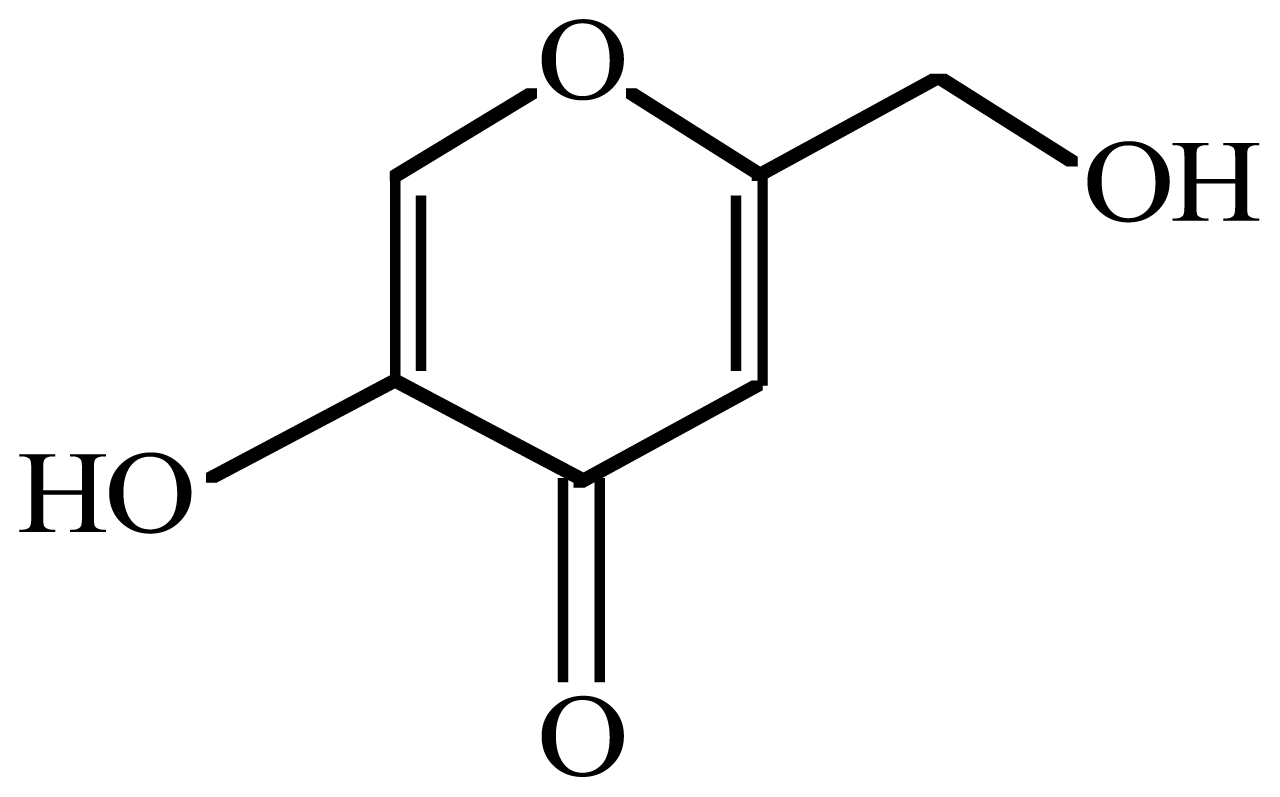
3.2. Chemicals
3.3. Antifungal Bioassay
3.3.1. Agar Plate Bioassay: Filamentous Fungi
3.3.2. Microtiter Plate (microdilution) Bioassay: Filamentous Fungi
3.3.3. Microtiter Plate (microdilution) Bioassay: Yeasts
4. Conclusions
Acknowledgments
References
- Bentley, R. From miso, saké and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep 2006, 23, 1046–1062. [Google Scholar]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci 2009, 10, 2440–2475. [Google Scholar]
- Leyden, J.J.; Shergill, B.; Micali, G.; Downie, J.; Wallo, W. Natural options for the management of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol 2011, 25, 1140–1145. [Google Scholar]
- Terabayashi, Y.; Sano, M.; Yamane, N.; Marui, J.; Tamano, K.; Sagara, J.; Dohmoto, M.; Oda, K.; Ohshima, E.; Tachibana, K.; et al. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet. Biol 2010, 47, 953–961. [Google Scholar]
- Oda, K.; Kobayashi, A.; Ohashi, S.; Sano, M. Aspergillus oryzae laeA regulates kojic acid synthesis genes. Biosci. Biotechnol. Biochem 2011, 75, 1832–1834. [Google Scholar]
- Niwa, Y.; Akamatsu, H. Kojic acid scavenges free radicals while potentiating leukocyte functions including free radical generation. Inflammation 1991, 15, 303–315. [Google Scholar]
- Rodrigues, A.P.; Carvalho, A.S.; Santos, A.S.; Alves, C.N.; do Nascimento, J.L.; Silva, E.O. Kojic acid, a secondary metabolite from Aspergillus sp., acts as an inducer of macrophage activation. Cell Biol. Int 2011, 35, 335–343. [Google Scholar]
- Chee, H.Y.; Lee, E.H. Fungistatic activity of kojic acid against human pathogenic fungi and inhibition of melanin-production in Cryptococcus neoformans. Mycobiology 2003, 31, 248–250. [Google Scholar]
- Reddy, B.V.; Reddy, M.R.; Madan, C.H.; Kumar, K.P.; Rao, M.S. Indium(III) chloride catalyzed three-component coupling reaction: A novel synthesis of 2-substituted aryl(indolyl)kojic acid derivatives as potent antifungal and antibacterial agents. Bioorg. Med. Chem. Lett 2010, 20, 7507–7511. [Google Scholar]
- Brtko, J.; Rondahl, L.; Ficková, M.; Hudecová, D.; Eybl, V.; Uher, M. Kojic acid and its derivatives: History and present state of art. Cent. Eur. J. Public Health 2004, 12, S16–S18. [Google Scholar]
- Mohamad, R.; Mohamed, M.S.; Suhaili, N.; Salleh, M.M.; Ariff, A.B. Kojic acid: Applications and development of fermentation process for production. Biotech. Mol. Biol. Rev 2010, 5, 24–37. [Google Scholar]
- Denning, D.W. Invasive aspergillosis. Clin. Infect. Dis 1998, 26, 781–805. [Google Scholar]
- El-Shanawany, A.A.; Mostafa, M.E.; Barakat, A. Fungal populations and mycotoxins in silage in Assiut and Sohag governorates in Egypt, with a special reference to characteristic Aspergilli toxins. Mycopathologia 2005, 159, 281–289. [Google Scholar]
- Frisvad, J.C.; Rank, C.; Nielsen, K.F.; Larsen, T.O. Metabolomics of Aspergillus fumigatus. Med. Mycol 2009, 47, S53–S71. [Google Scholar]
- Niimi, K.; Harding, D.R.; Parshot, R.; King, A.; Lun, D.J.; Decottignies, A.; Niimi, M.; Lin, S.; Cannon, R.D.; Goffeau, A.; et al. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a d-octapeptide derivative. Antimicrob. Agents Chemother 2004, 48, 1256–1271. [Google Scholar]
- Lavigne, J.P.; Brunel, J.M.; Chevalier, J.; Pages, J.M. Squalamine, an original chemosensitizer to combat antibiotic-resistant gram-negative bacteria. J. Antimicrob. Chemother 2010, 65, 799–801. [Google Scholar]
- Xue, T.; Nguyen, C.K.; Romans, A.; May, G.S. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryot. Cell 2004, 3, 557–560. [Google Scholar]
- Reyes, G.; Romans, A.; Nguyen, C.K.; May, G.S. Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryot. Cell 2006, 5, 1934–1940. [Google Scholar]
- Saccharomyces Genome Database. Available online: http://www.yeastgenome.org accessed on 5 September 2012.
- Clinical and Laboratory Standards Institute (CLSI), Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard, Second Edition ed; CLSI: Wayne, PA, USA, 2008; Volume 22.
- Odds, F. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother 2003, 52, 1. [Google Scholar]
- Statistics to Use. Available online: http://www.physics.csbsju.edu/stats/ accessed on 5 September 2012.
- Clemons, K.V.; Schwartz, J.A.; Stevens, D.A. Therapeutic and toxicologic studies in a murine model of invasive pulmonary aspergillosis. Med. Mycol 2011, 49, 834–847. [Google Scholar]
- Sokol-Anderson, M.L.; Brajtburg, J.; Medoff, G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis 1986, 154, 76–83. [Google Scholar]
- Graybill, J.R.; Burgess, D.S.; Hardin, T.C. Key issues concerning fungistatic versus fungicidal drugs. Eur. J. Clin. Microbiol. Infect. Dis 1997, 16, 42–50. [Google Scholar]
- An, M.; Shen, H.; Cao, Y.; Zhang, J.; Cai, Y.; Wang, R.; Jiang, Y. Allicin enhances the oxidative damage effect of amphotericin B against Candida albicans. Int. J. Antimicrob. Agents 2009, 33, 258–263. [Google Scholar]
- González-Párraga, P.; Sánchez-Fresneda, R.; Zaragoza, O.; Argüelles, J.C. Amphotericin B induces trehalose synthesis and simultaneously activates an antioxidant enzymatic response in Candida albicans. Biochim. Biophys. Acta 2011, 1810, 777–783. [Google Scholar]
- Okamoto, Y.; Aoki, S.; Mataga, I. Enhancement of amphotericin B activity against Candida albicans by superoxide radical. Mycopathologia 2004, 158, 9–15. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. the EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2. Clin. Microbiol. Infect 2012, 18, E246–E247. [Google Scholar]
- Heinisch, J.J.; Lorberg, A.; Schmitz, H.P.; Jacoby, J.J. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol 1999, 32, 671–680. [Google Scholar]
- Hahn, J.S.; Thiele, D.J. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem 2002, 277, 21278–21284. [Google Scholar]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; May, G.S. Targeting antioxidative signal transduction and stress response system: Control of pathogenic Aspergillus with phenolics that inhibit mitochondrial function. J. Appl. Microbiol 2006, 101, 181–189. [Google Scholar]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci 2002, 58, 649–662. [Google Scholar]
- Kojima, K.; Takano, Y.; Yoshimi, A.; Tanaka, C.; Kikuchi, T.; Okuno, T. Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol 2004, 53, 1785–1796. [Google Scholar]
- Kim, J.H.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; May, G.S.; Campbell, B.C. Chemosensitization of fungal pathogens to antimicrobial agents using benzo analogs. FEMS Microbiol. Lett 2008, 281, 64–72. [Google Scholar]
- Becker, W.F.; von Jagow, G.; Anke, T.; Steglich, W. Oudemansin, strobilurin A, strobilurin B, and myxothiazol: new inhibitors of the bc1 segment of the respiratory chain with an E-β-methoxyacrylate system as common structural element. FEBS Lett 1981, 132, 329–333. [Google Scholar]
- Takimoto, H.; Machida, K.; Ueki, M.; Tanaka, T.; Taniguchi, M. UK-2A, B, C and D, novel antifungal antibiotics from Streptomyces sp. 517–02. IV. Comparative studies of UK-2A with antimycin A3 on cytotoxic activity and reactive oxygen species generation in LLC-PK1 cells. J. Antibiot 1999, 52, 480–484. [Google Scholar]
- Vincent, J.M. Distortion of fungal hyphae in the presence of certain inhibitors. Nature 1947, 159, 850. [Google Scholar]
| Fungal strains | Strain characteristics | Source/Reference |
|---|---|---|
| Filamentous fungi | ||
| Aspergillus flavus 3357 | Kojic acid producer, Human pathogen (aspergillosis), Plant pathogen | NRRL a |
| A. parasiticus 5862 | Kojic acid producer, Plant pathogen | NRRL a |
| A. fumigatus AF293 | Human pathogen (aspergillosis), Reference clinical strain | [17] |
| A. fumigatus sakAΔ | Human pathogen (aspergillosis), MAPK mutant derived from AF293 | [17] |
| A. fumigatus mpkCΔ | Human pathogen (aspergillosis), MAPK mutant derived from AF293 | [18] |
| A. terreus UAB673 | Human pathogen (aspergillosis), Clinical isolate | CDC b |
| A. terreus UAB680 | Human pathogen (aspergillosis), Clinical isolate | CDC b |
| A. terreus UAB698 | Human pathogen (aspergillosis), Clinical isolate | CDC b |
| Yeasts | ||
| Candida albicans 90028 | Human pathogen (candidiasis), Reference clinical strain | ATCC c |
| C. albicans CAN276 | Human pathogen (candidiasis), Clinical isolate | IHMT d |
| C. krusei 6258 | Human pathogen (candidiasis), Reference clinical strain | ATCC c |
| C. krusei CAN75 | Human pathogen (candidiasis), Clinical isolate | IHMT d |
| C. tropicalis CAN286 | Human pathogen (candidiasis), Clinical isolate | IHMT d |
| Cryptococcus neoformans CN24 | Human pathogen (cryptococcosis), Clinical isolate | IHMT d |
| Saccharomyces cerevisiae BY4741 | Model yeast, Parental strain (Mat a his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) | SGD e |
| S. cerevisiae bck1Δ | MAPK mutant derived from BY4741 | SGD e |
| S. cerevisiae slt2Δ | MAPK kinase kinase mutant derived from BY4741 | SGD e |
| Strains | Compounds | MIC alone | MIC combined | FICI | MFC alone | MFC combined | FFCI |
|---|---|---|---|---|---|---|---|
| A. fumigatus | Kojic | >12.8 b | 0.8 | 0.5 | >12.8 | 0.8 | 0.5 |
| AF293 | H2O2 | 8 | 4 | 8 | 4 | ||
| A. fumigatus | Kojic | >12.8 | 12.8 | 1.0 | >12.8 | 12.8 | 1.0 |
| sakAΔ | H2O2 | 4 | 2 | 4 | 2 | ||
| A. fumigatus | Kojic | >12.8 | 12.8 | 1.0 | >12.8 | 12.8 | 1.0 |
| mpkCΔ | H2O2 | 4 | 2 | 4 | 2 | ||
| A. terreus | Kojic | >12.8 | 6.4 | 0.8 | >12.8 | 12.8 | 0.8 |
| UAB673 | H2O2 | 2 | 1 | 4 | 1 | ||
| A. terreus | Kojic | >12.8 | 6.4 | 0.8 | >12.8 | 12.8 | 1.0 |
| UAB680 | H2O2 | 2 | 1 | 2 | 1 | ||
| A. terreus | Kojic | >12.8 | 6.4 | 0.8 | >12.8 | 12.8 | 1.0 |
| UAB698 | H2O2 | 2 | 1 | 2 | 1 | ||
| Mean | Kojic | >12.8 | 7.6 | 0.8 | >12.8 | 10.8 | 0.9 |
| H2O2 | 3.7 | 1.8 | 4.0 | 1.8 | |||
| t-test | Kojic | - | p < 0.001 | - | - | p < 0.001 | - |
| H2O2 | p < 0.5 | p < 0.1 | |||||
| Strains | Compounds | MIC alone | MIC combined | FICI | MFC alone | MFC combined | FFCI |
|---|---|---|---|---|---|---|---|
| A. fumigatus | Kojic | >12.8 b | 0.8 | 0.8 | >12.8 | 3.2 | 0.6 |
| AF293 | AMB | 4 | 2 | >32 c | 32 | (99.8% inhibition) | |
| A. fumigatus | Kojic | >12.8 | 12.8 | 1.0 | >12.8 | 0.4 | 0.5 |
| sakAΔ | AMB | 2 | 1 | (85%–90% inhibition) | 4 | 2 | |
| A. fumigatus | Kojic | >12.8 | 0.2 | 0.5 | >12.8 | 0.2 | 0.5 |
| mpkCΔ | AMB | 4 | 2 | 8 | 4 | ||
| C. albicans | Kojic | >12.8 | 6.4 | 0.8 | >12.8 | > 12.8 | 2.0 |
| CAN276 | AMB | 1 | 0.5 | 1 | 1 | ||
| C. krusei | Kojic | >12.8 | 0.4 | 0.5 | >12.8 | 6.4 | 0.8 |
| ATCC 6258 | AMB | 2 | 1 | 2 | 1 | ||
| Cryptococcus | Kojic | >12.8 | 0.4 | 0.5 | >12.8 | 3.2 | 0.6 |
| neoformans CN24 | AMB | 2 | 1 | 2 | 1 | ||
| Mean | Kojic | >12.8 | 3.5 | 0.7 | >12.8 | 6.5 | 0.8 |
| AMB | 2.5 | 1.3 | 13.5 | 6.8 | |||
| t-test | Kojic | - | p < 0.001 | - | - | p < 0.005 | - |
| AMB | p < 0.05 | p < 1.0 | |||||
| Strains | Compounds | MIC alone | MIC combined | FICI | MFC alone | MFC combined | FFCI |
|---|---|---|---|---|---|---|---|
| S. cerevisiae | Kojic | >12.8 b | 6.4 | 0.8 | > 12.8 | 12.8 | 1.0 |
| BY4741 | AMB | 2 | 1 | 4 | 2 | ||
| S. cerevisiae | Kojic | >12.8 | 6.4 | 0.8 | > 12.8 | 12.8 | 1.0 |
| slt2Δ | AMB | 2 | 1 | 4 | 2 | ||
| S. cerevisiae | Kojic | >12.8 | 6.4 | 0.8 | > 12.8 | 12.8 | 1.0 |
| bck1Δ | AMB | 2 | 1 | 4 | 2 | ||
| Mean | Kojic | >12.8 | 6.4 | 0.8 | > 12.8 | 12.8 | 1.0 |
| AMB | 2 | 1 | 4 | 2 | |||
| t-test | Kojic | - | p < 0.001 | - | - | p < 0.001 | - |
| AMB | p < 0.001 | p < 0.001 | |||||
| Fungal strains | Agents co-applied | |
|---|---|---|
| H2O2 (FICI, FFCI) b | AMB (FICI, FFCI) b | |
| Filamentous fungi | ||
| Aspergillus flavus 3357 | - | - |
| A. parasiticus 5862 | - | - |
| A. fumigatus AF293 | + (0.5, 0.5) | + (0.8, 0.6) |
| A. fumigatus sakAΔ | + (1.0, 1.0) | + (1.0, 0.5) |
| A. fumigatus mpkCΔ | + (1.0, 1.0) | + (0.5, 0.5) |
| A. terreus UAB673 | + (0.8, 0.8) | - |
| A. terreus UAB680 | + (0.8, 1.0) | - |
| A. terreus UAB698 | + (0.8, 1.0) | - |
| Yeasts | ||
| Candida albicans 90028 | - | - |
| C. albicans CAN276 | - | + (0.8, 2.0) |
| C. krusei 6258 | - | + (0.5, 0.8) |
| C. krusei CAN75 | - | - |
| C. tropicalis CAN286 | - | - |
| Cryptococcus neoformans CN24 | - | + (0.5, 0.6) |
| Saccharomyces cerevisiae BY4741 | - | + (0.8, 1.0) |
| S. cerevisiae bck1Δ | - | + (0.8, 1.0) |
| S. cerevisiae slt2Δ | - | + (0.8, 1.0) |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, J.H.; Chang, P.-K.; Chan, K.L.; Faria, N.C.G.; Mahoney, N.; Kim, Y.K.; Martins, M.D.L.; Campbell, B.C. Enhancement of Commercial Antifungal Agents by Kojic Acid. Int. J. Mol. Sci. 2012, 13, 13867-13880. https://doi.org/10.3390/ijms131113867
Kim JH, Chang P-K, Chan KL, Faria NCG, Mahoney N, Kim YK, Martins MDL, Campbell BC. Enhancement of Commercial Antifungal Agents by Kojic Acid. International Journal of Molecular Sciences. 2012; 13(11):13867-13880. https://doi.org/10.3390/ijms131113867
Chicago/Turabian StyleKim, Jong H., Perng-Kuang Chang, Kathleen L. Chan, Natália C. G. Faria, Noreen Mahoney, Young K. Kim, Maria De L. Martins, and Bruce C. Campbell. 2012. "Enhancement of Commercial Antifungal Agents by Kojic Acid" International Journal of Molecular Sciences 13, no. 11: 13867-13880. https://doi.org/10.3390/ijms131113867






