Profiling the Proteome of Exhaled Breath Condensate in Healthy Smokers and COPD Patients by LC-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Pooling
2.2. Identification of Proteins with LC-MS/MS
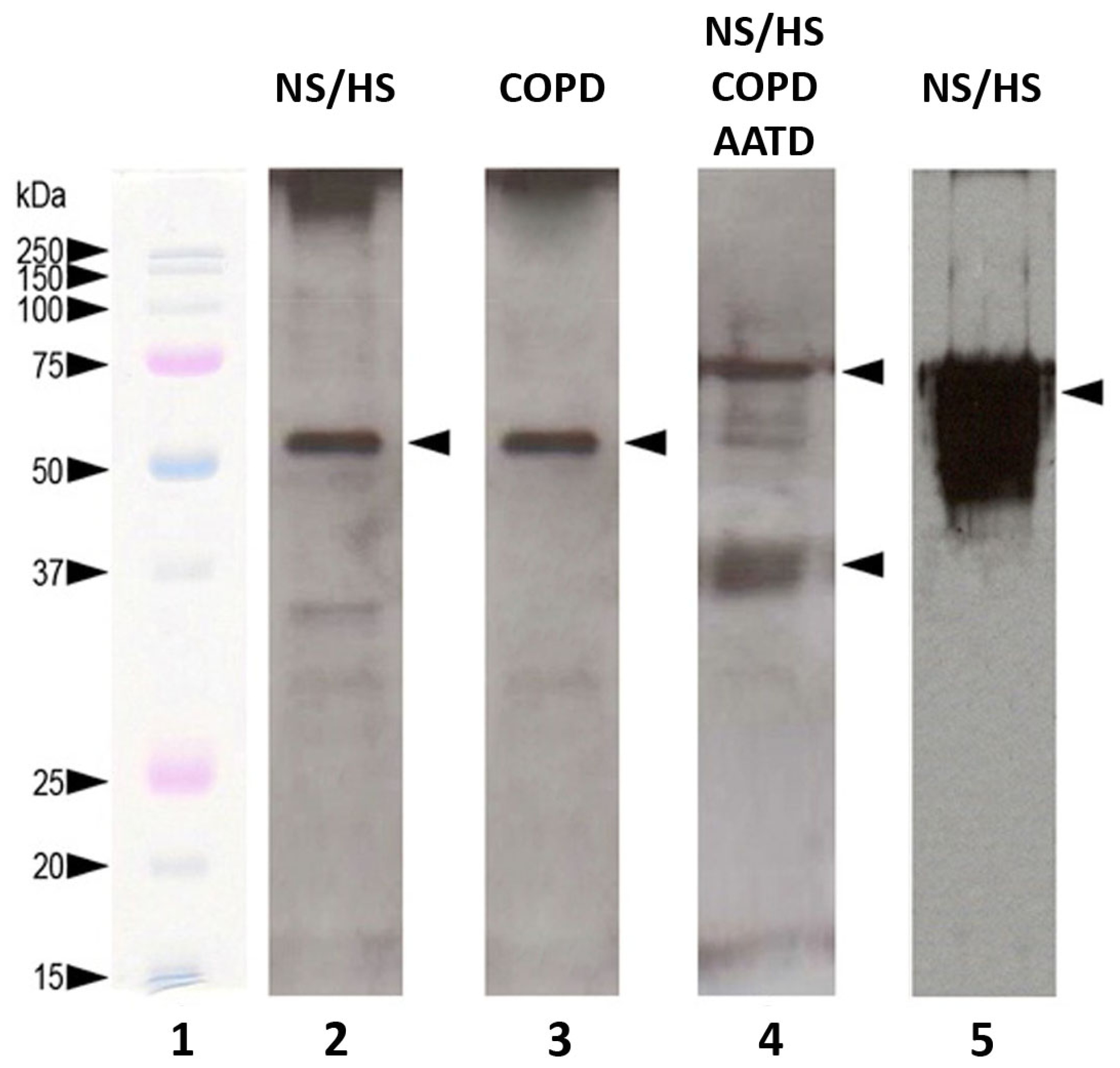
2.3. Western Blotting and SELDI-MS for Partial Validation of Data
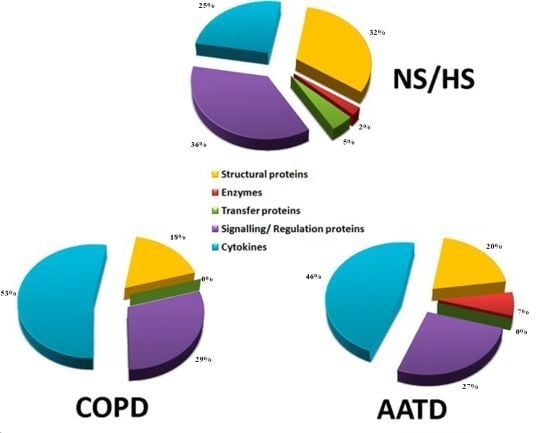
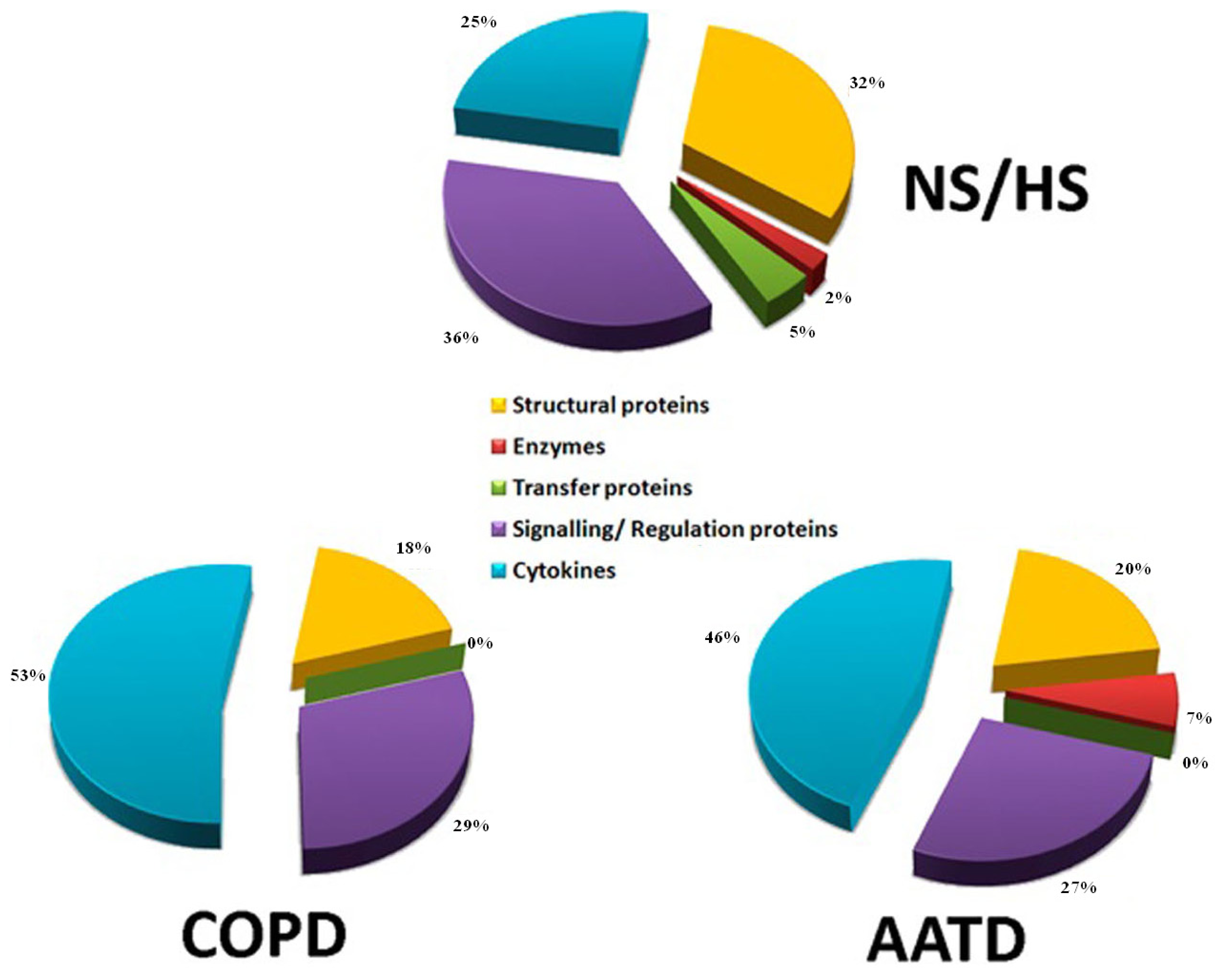
2.4. Identification of Proteins Contained in EBCs
2.5. Study Limitations
3. Experimental Section
3.1. Study Design and Study Population
3.2. EBC Collection and LC-MS/MS Analysis
3.3. One-D PAGE and Western Blotting
3.4. SELDI-TOF Analysis
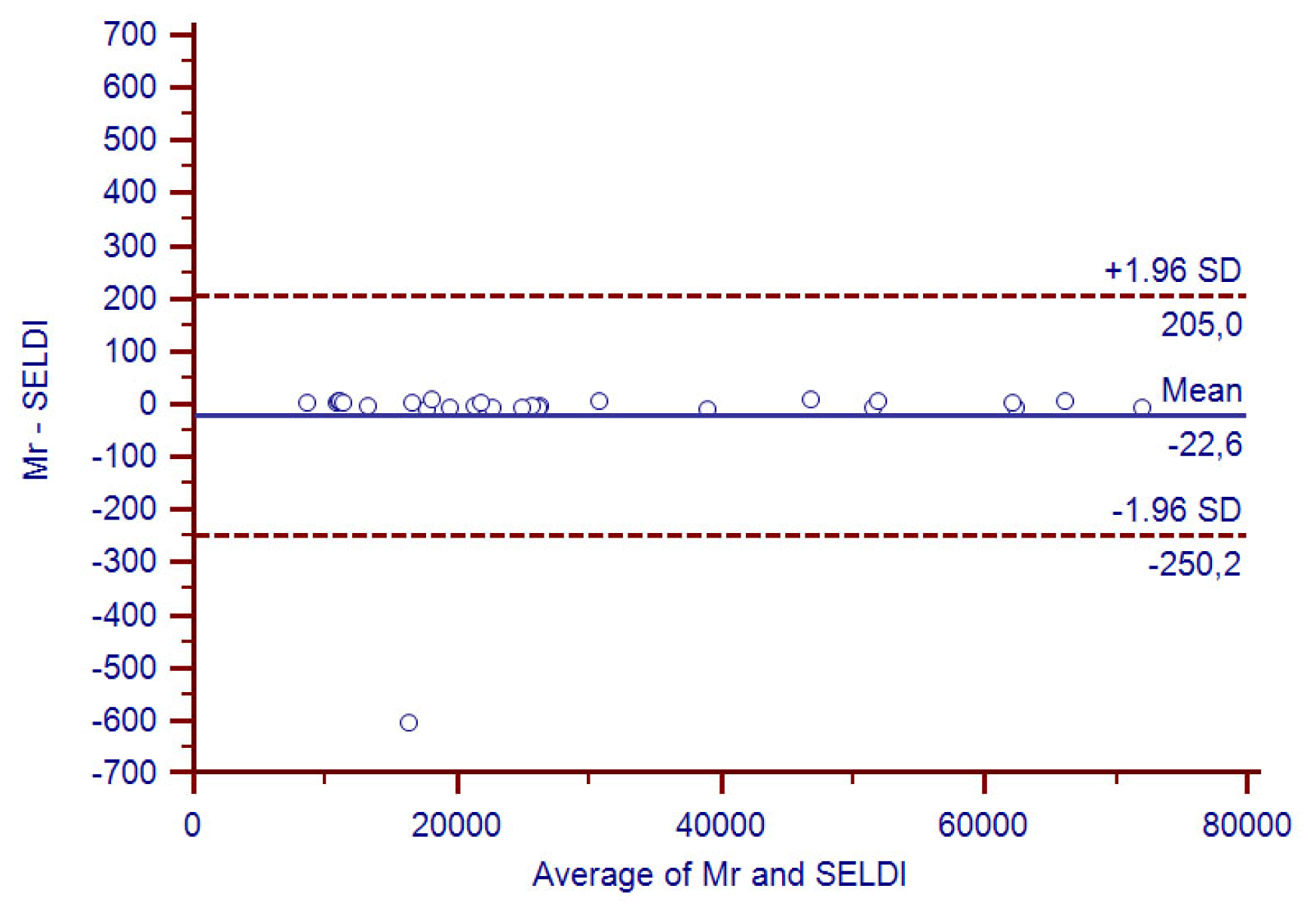
3.5. Reproducibility of the Study
4. Conclusions
Supplementary Information
ijms-13-13894-s001.pdfAcknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Viglio, S.; Iadarola, P.; Lupi, A.; Trisolini, R.; Tinelli, C.; Balbi, B.; Grassi, V.; Worlitzsch, D.; Döring, G.; Meloni, F.; et al. MEKC of desmosine and isodesmosine in urine of chronic destructive lung disease patients. Eur. Respir. J 2000, 15, 1039–1045. [Google Scholar]
- Slebos, D.J.; Scholma, J.; Boezen, H.M.; Koëter, G.H.; van der Bij, W.; Postma, D.S.; Kauffman, H.F. Longitudinal profile of bronchoalveolar lavage cell characteristics in patients with a good outcome after lung transplantation. Am. J. Respir. Crit. Care Med 2002, 165, 501–507. [Google Scholar]
- Sukoh, N.; Hizawa, N.; Yamamoto, H.; Suzuki, A. Increased neutrophils in bronchoalveolar lavage fluids from a patient with pulmonary edema associated with pheochromocytoma. Intern. Med 2004, 43, 1194–1197. [Google Scholar]
- Larsen, K.; Malmström, J.; Wildt, M.; Dahlqvist, C.; Hansson, L.; Marko-Varga, G.; Bjermer, L.; Scheja, A.; Westergren-Thorsson, G. Functional and phenotypical comparison of myofibroblasts derived from biopsies and bronchoalveolar lavage in mild asthma and scleroderma. Respir. Res 2006, 7, 11. [Google Scholar]
- Magi, B.; Bargagli, E.; Bini, L.; Rottoli, P. Proteome analysis of bronchoalveolar lavage in lung diseases. Proteomics 2006, 6, 6354–6369. [Google Scholar]
- Plymoth, A.; Löfdahl, C.G.; Ekberg-Jansson, A.; Dahlbäck, M.; Broberg, P.; Foster, M.; Fehniger, T.E.; Marko-Varga, G. Protein expression patterns associated with progression of chronic obstructive pulmonary disease in bronchoalveolar lavage of smokers. Clin. Chem 2007, 53, 636–644. [Google Scholar]
- Fahy, J.V.; Wong, H.; Liu, J.; Boushey, H.A. Comparison of samples collected by sputum induction and bronchoscopy from asthmatic and healthy subjects. Am. J. Respir. Crit. Care Med 1995, 152, 53–58. [Google Scholar]
- Pizzichini, E.; Pizzichini, M.M.; Efthimiadis, A.; Evans, S.; Morris, M.M.; Squillacem, D.; Gleichm, G.J.; Dolovich, J.; Hargreavem, F.E. Indices of airway inflammation in induced sputum: Reproducibility and validity of cell and fluid-phase measurements. Am. J. Respir. Crit. Care Med 1996, 154, 308–317. [Google Scholar]
- Jones, P.D.; Hankin, R.; Simpson, J.; Gibson, P.G.; Henry, R.L. The tolerability, safety, and success of sputum induction and combined hypertonic saline challenge in children. Am. J. Respir. Crit. Care Med 2001, 164, 1146–1149. [Google Scholar]
- Fahy, J.V.; Boushey, H.A.; Lazarus, S.C.; Mauger, E.A.; Cherniack, R.M.; Chinchilli, V.M.; Craig, T.J.; Drazen, J.M.; Ford, J.G.; Fish, J.E.; et al. NHLBI Asthma Clinical Research Network. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am. J. Respir. Crit. Care Med 2001, 163, 1470–1475. [Google Scholar]
- Casado, B.; Iadarola, P.; Pannell, L.K.; Luisetti, M.; Corsico, A.; Ansaldo, E.; Ferrarotti, I.; Boschetto, P.; Baraniuk, J.N. Protein expression in sputum of smokers and chronic obstructive pulmonary disease patients: a pilot study by CapLC-ESI-Q-TOF. J. Proteome Res 2007, 6, 4615–4623. [Google Scholar]
- Simpson, J.L.; Wood, L.G.; Gibson, P.G. Inflammatory mediators in exhaled breath, induced sputum and saliva. Clin. Exp. Allergy 2005, 35, 1180–1185. [Google Scholar]
- Jackson, A.S.; Sandrini, A.; Campbell, C.; Chow, S.; Thomas, P.S.; Yates, D.H. Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med 2007, 175, 222–227. [Google Scholar]
- Piotrowski, W.J.; Antczak, A.; Marczak, J.; Nawrocka, A.; Kurmanowska, Z.; Górski, P. Eicosanoids in exhaled breath condensate and BAL fluid of patients with sarcoidosis. Chest 2007, 132, 589–596. [Google Scholar]
- Mondino, C.; Ciabattoni, G.; Koch, P.; Pistelli, R.; Trové, A.; Barnes, P.J.; Montuschi, P. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J. Allergy Clin. Immunol 2004, 114, 761–767. [Google Scholar]
- Gianazza, E.; Allegra, L.; Bucchioni, E.; Eberini, I.; Puglisi, L.; Blasi, F.; Terzano, C.; Wait, R.; Sirtori, C.R. Increased keratin content detected by proteomic analysis of exhaled breath condensate from healthy persons who smoke. Am. J. Med 2004, 117, 51–54. [Google Scholar]
- Ko, F.W.S.; Lau, C.Y.K.; Leung, T.F.; Wong, G.W.K.; Lam, C.W.; Hui, D.S. Exhaled breath condensate levels of 8-isoprostane, growth related oncogene alpha and monocyte chemoattractant protein-1 in patients with chronic obstructive pulmonary disease. Respir. Med 2006, 100, 630–638. [Google Scholar]
- Bloemen, K.; Hooyberghs, J.; Desager, K.; Witters, E.; Schoeters, G. Non-invasive biomarker sampling and analysis of exhaled breath proteome. Proteomics Clin. Appl 2009, 3, 498–504. [Google Scholar]
- Antus, B.; Barta, I.; Czebe, K.; Horvath, I.; Csiszer, E. Analysis of cytokine pattern in exhaled breath condensate of lung transplant recipients with bronchiolitis obliterans syndrome. Inflamm. Res 2010, 59, 83–86. [Google Scholar]
- Bondesson, E.; Jansson, L.T.; Bengtsson, T.; Wollmer, P. Exhaled breath condensate-site and mechanisms of formation. J. Breath Res. 2009, 3. [Google Scholar] [CrossRef]
- Horváth, I.; Hunt, J.; Barnes, P.J.; Alving, K.; Antczak, A.; Baraldi, E.; Becher, G.; van Beurden, W.J.; Corradi, M.; Dekhuijzen, R.; et al. ATS/ERS Task Force on Exhaled Breath Condensate. Exhaled breath condensate: Methodological recommendations and unresolved questions. Eur. Respir. J 2005, 26, 523–548. [Google Scholar]
- Soyer, O.U.; Dizdar, E.A.; Keskin, O.; Lilly, C.; Kalayci, O. Comparison of two methods for exhaled breath condensate collection. Allergy 2006, 61, 1016–1018. [Google Scholar]
- Rosias, P.P.; Robroeks, C.M.; Kester, A.; den Hartog, G.J.; Wodzig, W.K.; Rijkers, G.T.; Zimmermann, L.J.; van Schayck, C.P.; Jöbsis, Q.; Dompeling, E. Biomarker reproducibility in exhaled breath condensate collected with different condensers. Eur. Respir. J 2008, 3, 934–942. [Google Scholar]
- Kurova, V.S.; Anaev, E.C.; Kononikhin, A.S.; Fedorchenko, K.Y.; Popov, I.A.; Kalupov, T.L.; Bratanov, D.O.; Nikolaev, E.N.; Varfolomeev, S.D. Proteomics of exhaled breath: Methodological nuances and pitfalls. Clin. Chem. Lab Med 2009, 47, 706–712. [Google Scholar]
- Borrill, Z.L.; Roy, K.; Singh, D. Exhaled breath condensate biomarkers in COPD. Eur. Respir. J 2008, 32, 472–486. [Google Scholar]
- Cao, W.; Duan, Y. Breath analysis: Potential for clinical diagnosis and exposure assessment. Clin. Chem 2006, 52, 800–811. [Google Scholar]
- Fumagalli, M.; Dolcini, L.; Sala, A.; Stolk, J.; Fregonese, L.; Ferrari, F.; Viglio, S.; Luisetti, M.; Iadarola, P. Proteomic analysis of exhaled breath condensate from single patients with pulmonary emphysema associated to α1-antitrypsin deficiency. J. Proteomics 2008, 71, 211–221. [Google Scholar]
- AmiGO—Gene Ontology. Available online: http://amigo.geneontology.org/cgi-bin/amigo/go.cgi accessed on 20 October 2011.
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res 1999, 8, 135–160. [Google Scholar]
- Gessner, C.; Dihazi, H.; Brettschneider, S.; Hammerschmidt, S.; Kuhn, H.; Eschrich, K.; Keller, T.; Engelmann, L.; Sack, U.; Wirtz, H. Presence of cytokeratins in exhaled breath condensate of mechanical ventilated patients. Respir. Med 2008, 102, 299–306. [Google Scholar]
- Hoffmann, H.J.; Tabaksblat, L.M.; Enghild, J.J.; Dahl, R. Human skin keratins are the major proteins in exhaled breath condensate. Eur. Respir. J 2008, 31, 380–384. [Google Scholar]
- Blobel, G.A.; Moll, R.; Franke, W.W.; Vogt-Moykopf, I. Cytokeratins in normal lung and lung carcinomas. I. Adenocarcinomas, squamous vell carcinomas and cultured cell lines. Virchows Arch. B 1984, 45, 407–429. [Google Scholar]
- Kharitonov, S.A.; Barnes, P.J. Exhaled markers of inflammation. Curr. Opin. Allergy Clin. Immunol 2001, 1, 217–224. [Google Scholar]
- Bucchioni, E.; Kharitonov, S.A.; Allegra, L.; Barnes, P.J. High levels of interleukin-6 in the exhaled breath condensate of patients with COPD. Respir. Med 2003, 97, 1299–1302. [Google Scholar]
- Garey, K.W.; Neuhauser, M.M.; Robbins, R.A.; Danziger, L.H.; Rubinstein, I. Markers of inflammation in exhaled breath condensate of young healthy smokers. Chest 2004, 125, 22–26. [Google Scholar]
- Leung, T.F.; Wong, G.W.; Ko, F.W.; Li, C.Y.; Yung, E.; Lam, C.W.; Fok, T.F. Analysis of growth factors and inflammatory cytokines in exhaled breath condensate from asthmatic children. Int. Arch. Allergy Immunol 2005, 137, 66–72. [Google Scholar]
- Gessner, C.; Scheibe, R.; Wötzel, M.; Hammerschmidt, S.; Kuhn, H.; Engelmann, L.; Hoheisel, G.; Gillissen, A.; Sack, U.; Wirtz, H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir. Med 2005, 99, 1229–1240. [Google Scholar]
- Sack, U.; Scheibe, R.; Wötzel, M.; Hammerschmidt, S.; Kuhn, H.; Emmrich, F.; Hoheisel, G.; Wirtz, H.; Gessner, C. Multiplex analysis of cytokines in exhaled breath condensate. Cytometry A 2006, 69, 169–172. [Google Scholar]
- Montuschi, P. Exhaled breath condensate in patients with COPD. Clin. Chim. Acta 2005, 356, 22–34. [Google Scholar]
- Koczulla, A.R.; Noeske, S.; Herr, C.; Koepke, J.; Jörres, R.A.; Nell, C.; Schmid, S.; Vogelmeier, C.; Bals, R. α-1 antitrypsin is elevated in exhaled breath condensate and serum in exacerbated COPD patients. Respir. Med 2012, 106, 120–126. [Google Scholar]
- Gomes-Alves, P.; Imrie, M.; Gray, R.D.; Nogueira, P.; Ciordia, S.; Pacheco, P.; Azevedo, P.; Lopes, C.; de Almeida, A.B.; Guardiano, M.; et al. SELDI-TOF biomarker signatures for cystic fibrosis, asthma and chronic obstructive pulmonary disease. Clin. Biochem 2010, 43, 168–177. [Google Scholar]
- Benabdeslam, H.; Abidi, H.; Garcia, I.; Bellon, G.; Gilly, R.; Revol, A. Lipid peroxidation and antioxidant defences in cystic fibrosis patients. Clin. Chem. Lab. Med 1999, 37, 511–516. [Google Scholar]
- Lanzetti, M.; da Costa, C.A.; Nesi, R.T.; Barroso, M.V.; Martins, V.; Victoni, T.; Lagente, V.; Pires, K.M.; Silva, P.M.E.; Resende, A.C.; et al. Oxidative stress and nitrosative stress are involved in different stages of proteolytic pulmonary emphysema. Free Radic. Biol. Med. 2012, in press. [Google Scholar]
- Vucević, D.; Radosavljević, T.; Zunić, S.; Dordević-Denić, G.; Pesić, B.C.; Radak, D. The role of oxidative stress in the pathogenesis of pulmonary emphysema. Med. Pregl 2005, 58, 472–477. [Google Scholar]
- Miniati, M.; Monti, S.; Stolk, J.; Mirarchi, G.; Falaschi, F.; Rabinovich, R.; Canapini, C.; Roca, J.; Rabe, K.F. Value of chest radiography in phenotyping chronic obstructive pulmonary disease. Eur. Respir. J 2008, 31, 509–515. [Google Scholar]
- Matrix Science-Mascot. Available online: http://www.matrixscience.com/search_form_select.html accessed on 30 May 2012.

| Subjects | Smoking status | Age (Years) | FEV1* (L) | FEV1 (%) | FVC ** (L) | FEV1/FVC (%) | Protein concentration in EBC (μg/mL) |
|---|---|---|---|---|---|---|---|
| NS (n = 25; F/M = 13/12) | Never-smokers | 33 ± 4.5 | 3.05 ± 0.67 | 91.52 ± 2.92 | 3.32 ± 0.79 | 75 ± 3.37 | 6.8 ± 0.7 |
| HS (n = 20; F/M = 10/10) | Smokers (8 p/y) | 35 ± 4.0 | 2.94 ± 0.36 | 89.45 ± 4.28 | 3.16 ± 0.51 | 72 ± 2.95 | 7.1 ± 0.9 |
| COPD (n = 15; F/M = 8/7) | Ex-smokers | 65 ± 8.0 | 1.56 ± 0.39 | 47.44 ± 9.37 | 1.84 ± 0.92 | 45 ± 9.42 | 19.3 ± 0.5 |
| AATD (n = 23; F/M = 12/11) | Ex-smokers | 40 ± 2.5 | 1.34 ± 0.51 | 45.92 ± 3.71 | 1.57 ± 0.58 | 43 ± 5.67 | 21.7 ± 0.4 |
| # | Description | Accession # | Mr (Da) | ESI Ion-trap/Nano ESI orbitrap | Presence in | ||||
|---|---|---|---|---|---|---|---|---|---|
| Coverage% | Query | MOWSE score% | NS/HS | COPD | AATD | ||||
| 1 | Type II, CK 1 | P04264 | 66039 | 16/25 | 12/20 | 99/99 | √ | — | — |
| 2 | Type II, CK 2 | P35908 | 65433 | 5/24 | 4/15 | 91/99 | √ | — | — |
| 3 | Type II, CK 5 | P13647 | 62378 | 34/23 | 119/13 | 99/99 | √ | — | — |
| 4 | Type II, CK 6B | P04259 | 60067 | 35/55 | 99/96 | 99/99 | √ | — | — |
| 5 | Type I, CK 9 | P35527 | 62064 | 42/11 | 101/10 | 99/99 | √ | — | — |
| 6 | Type I, CK 10 | P13645 | 58827 | 44/19 | 69/15 | 99/99 | √ | — | — |
| 7 | Type I, CK 14 | P02533 | 51562 | 36/12 | 58/9 | 99/99 | √ | — | — |
| 8 | Type I, CK 26 | Q7Z3Y9 | 51911 | 37/12 | 68/5 | 99/82 | √ | — | — |
| 9 | ACTC1 | P68032 | 42019 | 24/5 | 48/2 | 67/90 | √ | — | — |
| 10 | PS-A1 | Q8IWL2 | 26242 | 30/3 | 12/2 | 98/93 | √ | √ | √ |
| 11 | PS-A2 | Q8IWL1 | 26182 | 39/2 | 14/1 | 98/90 | √ | √ | √ |
| 12 | HSPG | P98160 | 468803 | 12/4 | 60/22 | 98/99 | √ | — | — |
| 13 | LAMB4 | B4DX23 | 76746 | 39/10 | 161/9 | 99/90 | √ | — | — |
| 14 | Histone H1.5 | P16401 | 22580 | 85/32 | 197/8 | 99/99 | √ | √ | √ |
| 15 | Alpha-1-AT | P01009 | 46737 | 41/5 | 24/2 | 97/82 | √ | √ | — |
| 16 | Calgranulin A | P05109 | 10835 | 68/16 | 18/2 | 61/76 | √ | √ | √ |
| 17 | Calgranulin B | P06702 | 13242 | 16/15 | 8/2 | 51/72 | √ | √ | √ |
| 18 | Erythropoietin | P01588 | 21307 | 20/5 | 14/2 | 98/83 | √ | — | — |
| 19 | Protein AMBP | P02760 | 39000 | 22/15 | 20/8 | 59/85 | √ | — | — |
| 20 | Ubiquitin | P62988 | 8565 | 43/16 | 8/3 | 92/81 | √ | — | — |
| 21 | Cystatin | P01040 | 11006 | 20/20 | 7/3 | 36/63 | √ | √ | √ |
| 22 | CSAct | P07602 | 58113 | 24/11 | 10/5 | 99/88 | √ | — | — |
| 23 | LAMP2 | P13473 | 44961 | 18/16 | 8/10 | 24/99 | √ | — | — |
| 24 | Kininogen 1 | P01042 | 71957 | 31/17 | 41/12 | 99/91 | √ | — | — |
| 25 | Complement C3 | P01024 | 187147 | 27/6 | 76/11 | 99/99 | √ | — | — |
| 26 | Nucleolar protein 4 | O94818 | 58419 | 23/4 | 117/3 | 99/95 | √ | — | — |
| 27 | VSIG8 | Q5VU13 | 43891 | 18/6 | 46/1 | 96/90 | √ | √ | √ |
| 28 | THRAP3 | Q9Y2W1 | 108666 | 57/36 | 421/44 | 99/99 | √ | — | — |
| 29 | CHD1 | O14646 | 196688 | 41/20 | 432/45 | 99/99 | √ | — | — |
| 30 | ZC3H4 | Q9UPT8 | 140257 | 23/11 | 240/21 | 99/99 | √ | — | — |
| 31 | MCP-1 | P13500 | 11025 | 38/7 | 12/3 | 95/85 | √ | √ | √ |
| 32 | IFN α-1/13 | P01562 | 21725 | 17/9 | 13/6 | 96/86 | √ | √ | — |
| 33 | IFN γ | P01579 | 19348 | 36/22 | 27/18 | 99/93 | √ | √ | √ |
| 34 | TNF | P01375 | 25644 | 20/11 | 7/5 | 28/64 | √ | √ | √ |
| 35 | GRO-α | P09341 | 11301 | 73/71 | 19/9 | 85/99 | √ | √ | √ |
| 36 | IL-1 α | P01583 | 30607 | 15/— | 8/— | 90/— | √ | — | √ |
| 37 | IL-1 β | P01584 | 30748 | 28/7 | 23/3 | 94/60 | √ | √ | — |
| 38 | IL-2 | P60568 | 17628 | 32/9 | 10/5 | 99/79 | √ | √ | √ |
| 39 | IL-12 subunit α | P29459 | 24874 | 30/8 | 10/2 | 92/80 | √ | √ | — |
| 40 | IL-12 subunit β | P29460 | 37169 | 30/15 | 26/13 | 98/83 | √ | — | — |
| 41 | IL-15 | P40933 | 18086 | 7/— | 2/— | 52/— | √ | √ | √ |
| 42 | Hb subunit β | P68871 | 15998 | 2/16 | 1/2 | 90/88 | √ | — | — |
| 43 | Serum Albumin | P02768 | 69367 | 34/25 | 44/13 | 98/94 | √ | — | — |
| 44 | Lysozyme C | P61626 | 16537 | 42/13 | 31/6 | 99/94 | √ | — | √ |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fumagalli, M.; Ferrari, F.; Luisetti, M.; Stolk, J.; Hiemstra, P.S.; Capuano, D.; Viglio, S.; Fregonese, L.; Cerveri, I.; Corana, F.; et al. Profiling the Proteome of Exhaled Breath Condensate in Healthy Smokers and COPD Patients by LC-MS/MS. Int. J. Mol. Sci. 2012, 13, 13894-13910. https://doi.org/10.3390/ijms131113894
Fumagalli M, Ferrari F, Luisetti M, Stolk J, Hiemstra PS, Capuano D, Viglio S, Fregonese L, Cerveri I, Corana F, et al. Profiling the Proteome of Exhaled Breath Condensate in Healthy Smokers and COPD Patients by LC-MS/MS. International Journal of Molecular Sciences. 2012; 13(11):13894-13910. https://doi.org/10.3390/ijms131113894
Chicago/Turabian StyleFumagalli, Marco, Fabio Ferrari, Maurizio Luisetti, Jan Stolk, Pieter S. Hiemstra, Daniela Capuano, Simona Viglio, Laura Fregonese, Isa Cerveri, Federica Corana, and et al. 2012. "Profiling the Proteome of Exhaled Breath Condensate in Healthy Smokers and COPD Patients by LC-MS/MS" International Journal of Molecular Sciences 13, no. 11: 13894-13910. https://doi.org/10.3390/ijms131113894