Molecular Signaling Involved in Oxysterol-Induced β1-Integrin Over-Expression in Human Macrophages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of β1-Integrin in Human Atherosclerotic Plaques
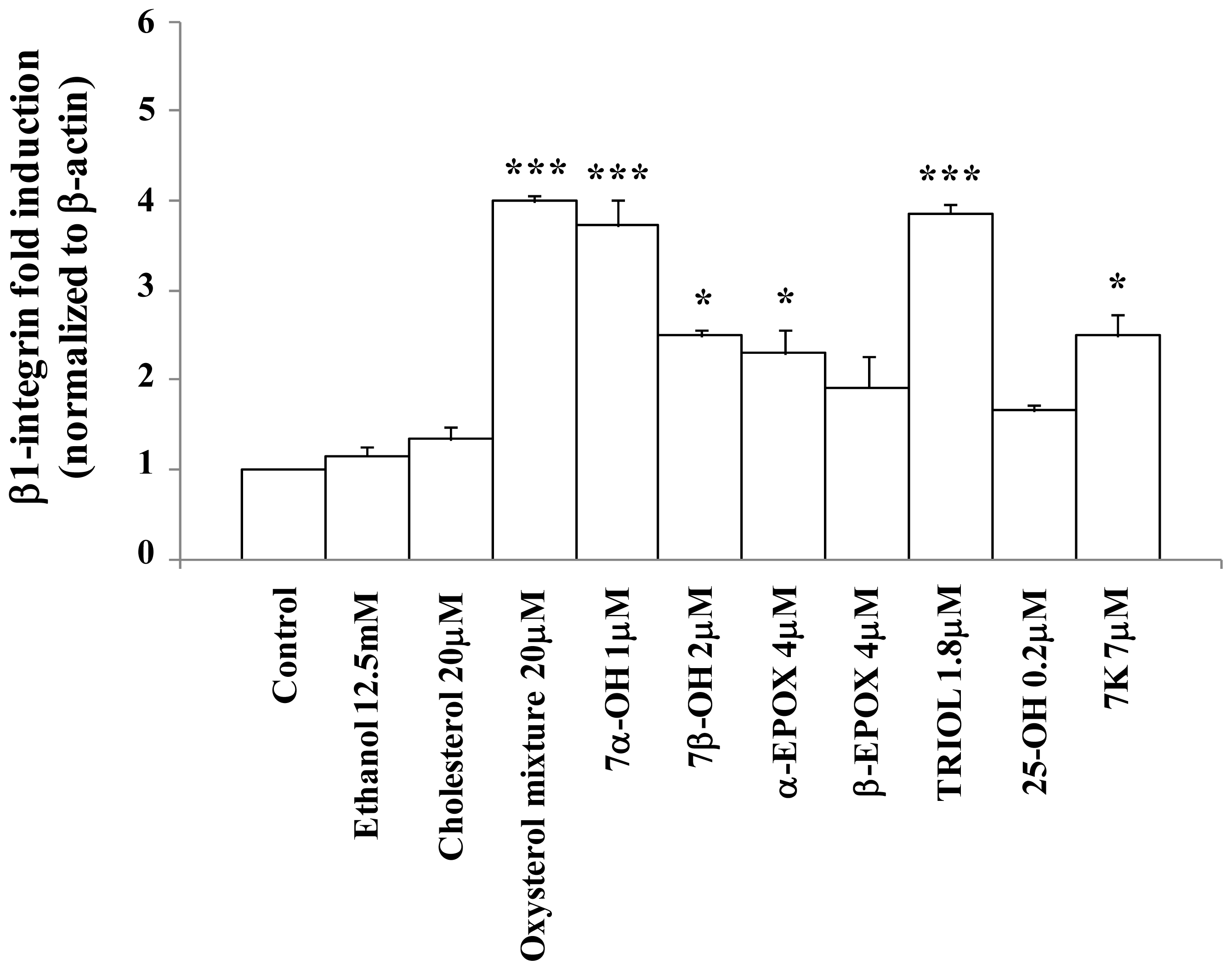
2.2. Oxysterols up-Regulate β1-Integrin in Human Promonocytic U937 Cells
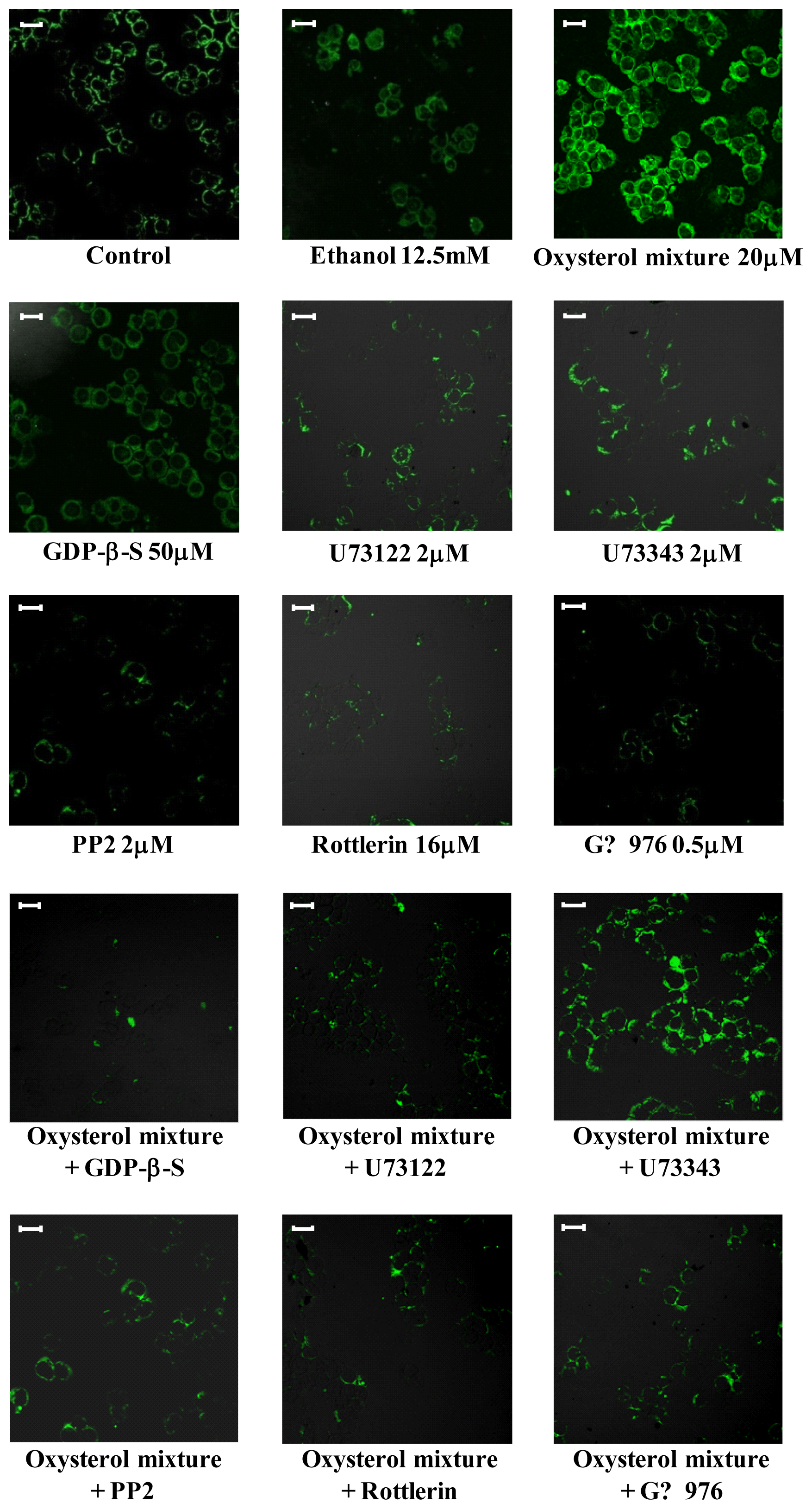
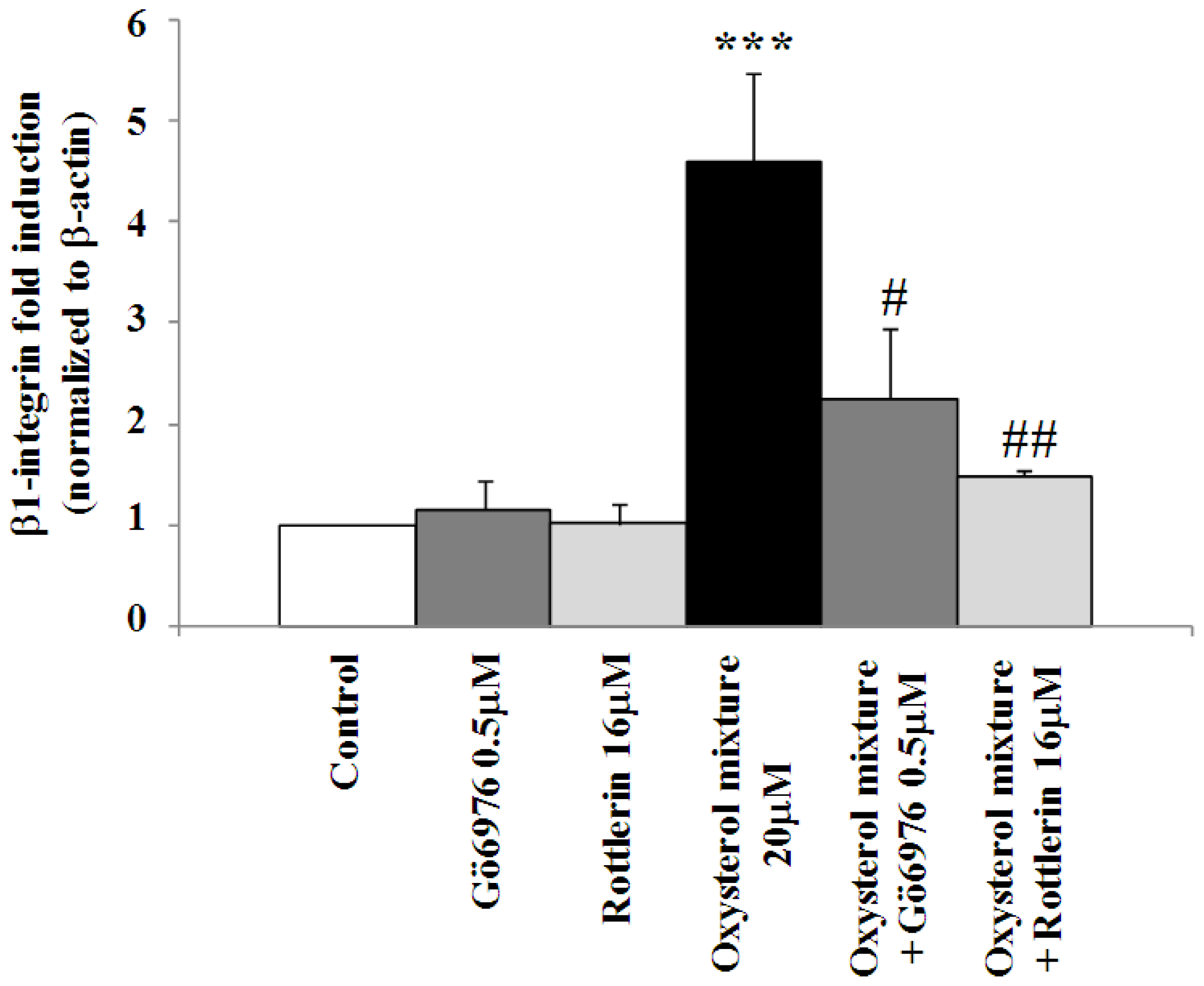
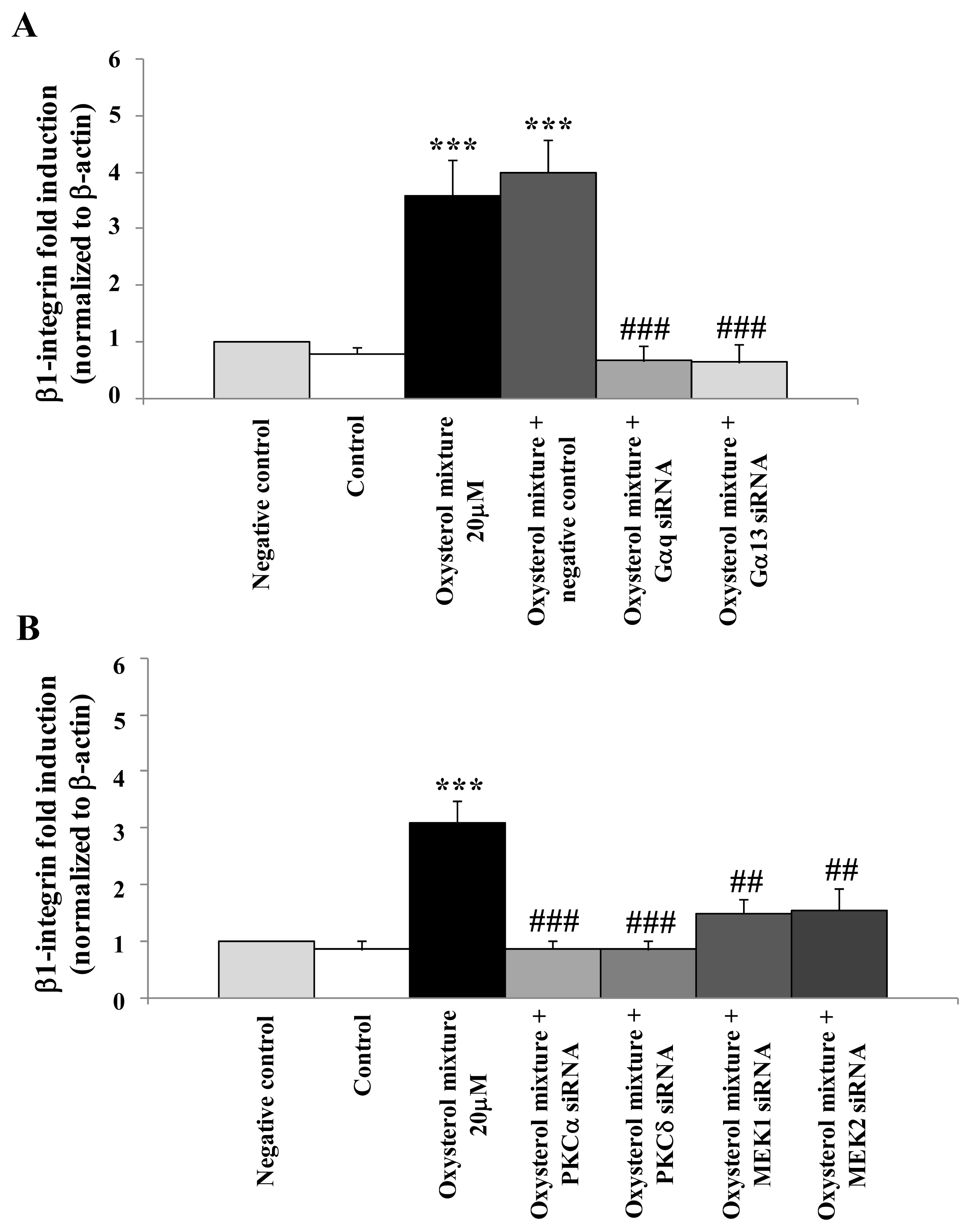
2.3. Involvement of the G Protein/Src/PLC/PKC Signaling Pathway in Oxysterol-Mediated Up-Regulation of β1-Integrin Levels
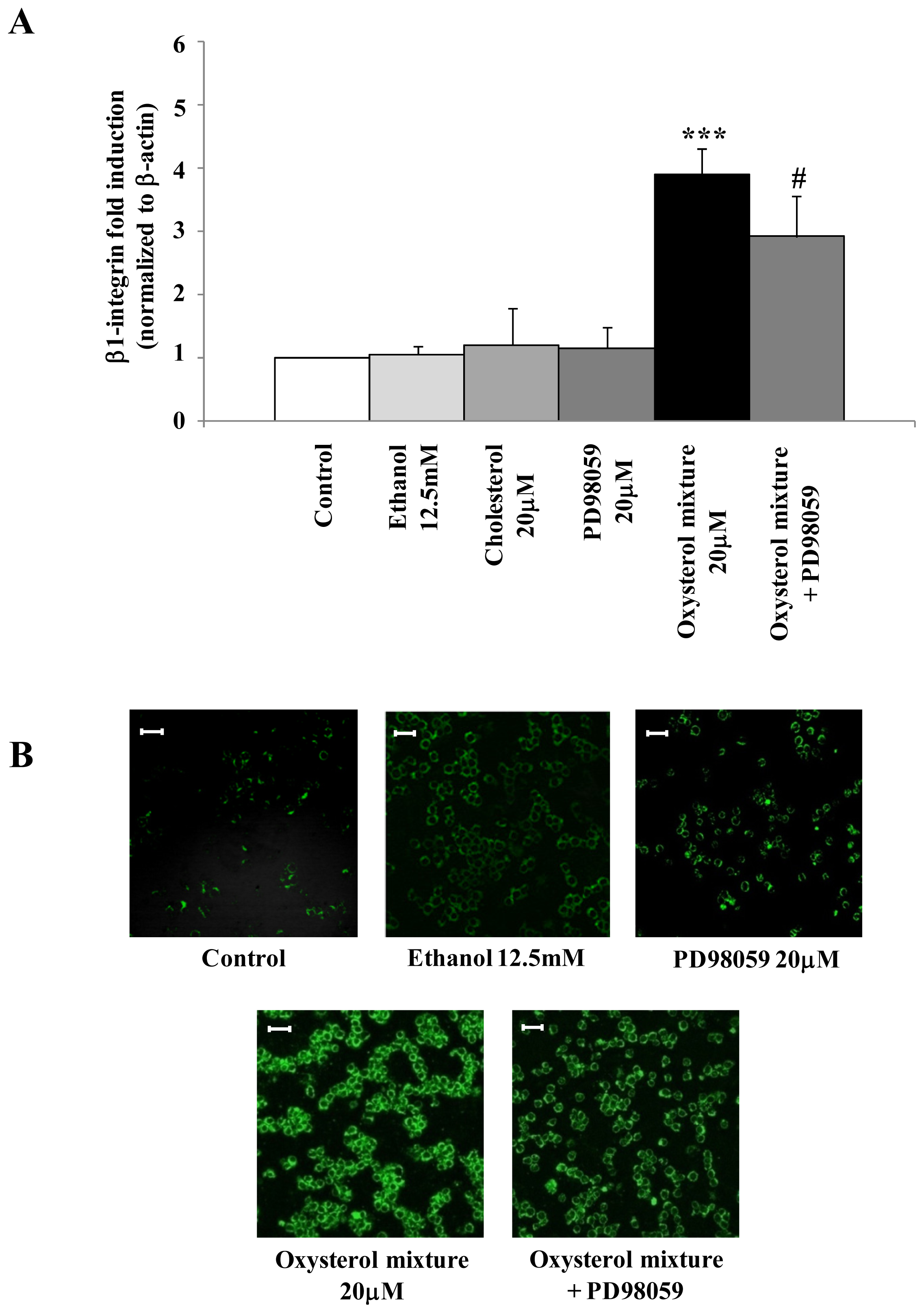
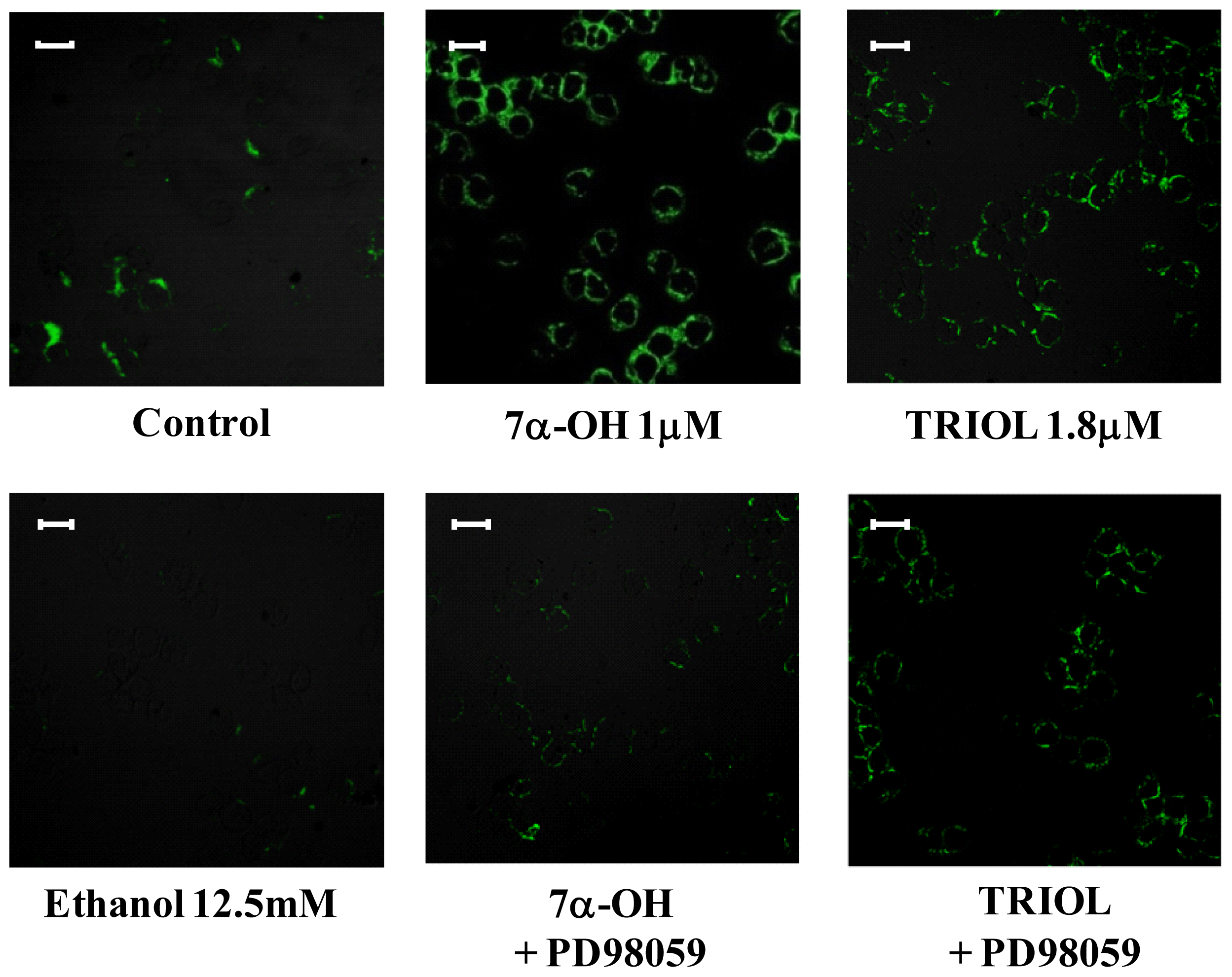
2.4. Involvement of the ERK1/2 Signaling Pathway in Up-Regulating β1-Integrin in U937 Promonocytic Cells
2.5. Prevention of β1-Integrin Over-Expression in U937 Cells Treated with the Oxysterol Mixture by Cell Transfection with Specific siRNAs for the G Protein/PKC/ERK Pathway
2.6. Discussion
3. Materials and Methods
3.1. Immunohistochemical Detection of β1-Integrin in Human Carotid Plaques
3.2. Cell Culture and Treatments
3.3. RNA Extraction
3.4. cDNA Preparation and Real-Time RT-PCR
3.5. siRNA Transfection
3.6. Analysis of β1-Integrin by Confocal Laser Microscopy
3.7. Statistical Analysis
4. Conclusion
Acknowledgements
- Conflict of InterestThe authors declare no conflict of interest.
References
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar]
- Alon, R.; Feigelson, S. From rolling to arrest on blood vessels: Leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin. Immunol 2002, 14, 93–104. [Google Scholar]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol 2007, 7, 678–689. [Google Scholar]
- Steinberg, D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat. Med 2002, 8, 1211–1217. [Google Scholar]
- Steinberg, D. Hypercholesterolemia and inflammation in atherogenesis: Two sides of the same coin. Mol. Nutr. Food Res 2005, 49, 995–998. [Google Scholar]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med 2011, 17, 1410–1422. [Google Scholar]
- Cave, A.C.; Brewer, A.C.; Narayanapanicker, A.; Ray, R.; Grieve, D.J.; Walker, S.; Shah, A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal 2006, 8, 691–728. [Google Scholar]
- Ting, H.J.; Stice, J.P.; Schaff, U.Y.; Hui, D.Y.; Rutledge, J.C.; Knowlton, A.A.; Passerini, A.G.; Simon, S.I. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-alpha. Circ. Res 2007, 100, 381–390. [Google Scholar]
- Wu, H.; Gower, R.M.; Wang, H.; Perrard, X.Y.; Ma, R.; Bullard, D.C.; Burns, A.R.; Paul, A.; Smith, C.W.; Simon, S.I.; et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 2009, 119, 2708–2717. [Google Scholar]
- Krieglstein, C.F.; Granger, D.N. Adhesion molecules and their role in vascular disease. Am. J. Hypertens 2001, 14, 44S–54S. [Google Scholar]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar]
- Margadant, C.; Monsuur, H.N.; Norman, J.C.; Sonnenberg, A. Mechanisms of integrin activation and trafficking. Curr. Opin. Cell. Biol 2011, 23, 607–614. [Google Scholar]
- Carragher, N.O.; Frame, M.C. Focal adhesion and actin dynamics: A place where kinases and proteases meet to promote invasion. Trends Cell Biol 2004, 14, 241–249. [Google Scholar]
- Barillari, G.; Albonici, L.; Incerpi, S.; Bogetto, L.; Pistritto, G.; Volpi, A.; Ensoli, B.; Manzari, V. Inflammatory cytokines stimulate vascular smooth muscle cells locomotion and growth by enhancing alpha5beta1 integrin expression and function. Atherosclerosis 2001, 154, 377–385. [Google Scholar]
- Wang, L.; Zheng, J.; Du, Y.; Huang, Y.; Li, J.; Liu, B.; Liu, C.J.; Zhu, Y.; Gao, Y.; Xu, Q.; et al. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ. Res 2010, 106, 514–525. [Google Scholar]
- Ferri, N.; Colombo, G.; Ferrandi, C.; Raines, E.W.; Levkau, B.; Corsini, A. Simvastatin reduces MMP1 expression in human smooth muscle cells cultured on polymerized collagen by inhibiting Rac1 activation. Arterioscler. Thromb. Vasc. Biol 2007, 27, 1043–1049. [Google Scholar]
- Del Carlo, M.; Schwartz, D.; Erickson, E.A.; Loeser, R.F. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic. Biol. Med 2007, 42, 1350–1358. [Google Scholar]
- Troussard, A.A.; Costello, P.; Yoganathan, T.N.; Kumagai, S.; Roskelley, C.D.; Dedhar, S. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9). Oncogene 2000, 19, 5444–5452. [Google Scholar]
- Su, G.; Atakilit, A.; Li, J.T.; Wu, N.; Bhattacharya, M.; Zhu, J.; Shieh, J.E.; Li, E.; Chen, R.; Sun, S.; et al. Absence of integrin αvβ3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am. J. Respir. Crit. Care Med 2012, 185, 58–66. [Google Scholar]
- Berliner, J.A.; Heinecke, J.W. The role of oxidized lipoproteins in atherogenesis. Free Radic. Biol. Med 1996, 20, 707–727. [Google Scholar]
- Badimon, L.; Storey, R.F.; Vilahur, G. Update on lipids, inflammation and atherothrombosis. Thromb. Haemost 2011, 105, S34–S42. [Google Scholar]
- Leonarduzzi, G.; Arkan, M.C.; Başağa, H.; Chiarpotto, E.; Sevanian, A.; Poli, G. Lipid oxidation products in cell signaling. Free Radic. Biol. Med 2000, 28, 1370–1378. [Google Scholar]
- Mazière, C.; Mazière, J.C. Activation of transcription factors and gene expression by oxidized low-density lipoprotein. Free Radic. Biol. Med 2009, 46, 127–137. [Google Scholar]
- Leonarduzzi, G.; Sottero, B.; Poli, G. Oxidized products of cholesterol: Dietary and metabolic origin, and proatherosclerotic effects. J. Nutr. Biochem 2002, 13, 700–710. [Google Scholar]
- Leonarduzzi, G.; Sottero, B.; Verde, V.; Poli, G. Oxidized Products of Cholesterol: Toxic Effect. In Reviews in Food and Nutrition Toxicity; Preedy, V.R., Watson, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 129–164. [Google Scholar]
- Leonarduzzi, G.; Chiarpotto, E.; Biasi, F.; Poli, G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol. Nutr. Food Res 2005, 49, 1044–1049. [Google Scholar]
- Poli, G.; Sottero, B.; Gargiulo, S.; Leonarduzzi, G. Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol. Aspects Med 2009, 30, 180–189. [Google Scholar]
- Sottero, B.; Gamba, P.; Gargiulo, S.; Leonarduzzi, G.; Poli, G. Cholesterol oxidation products and disease: An emerging topic of interest in medicinal chemistry. Curr. Med. Chem 2009, 16, 685–705. [Google Scholar]
- Vejux, A.; Lizard, G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Aspects Med 2009, 30, 153–170. [Google Scholar]
- Gargiulo, S.; Sottero, B.; Gamba, P.; Chiarpotto, E.; Poli, G.; Leonarduzzi, G. Plaque oxysterols induce unbalanced up-regulation of matrix metalloproteinase-9 in macrophagic cells through redox-sensitive signaling pathways: Implications regarding the vulnerability of atherosclerotic lesions. Free Radic. Biol. Med 2011, 51, 844–855. [Google Scholar]
- Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Biasi, F.; Poli, G. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free Radic. Biol. Med 2012, 52, 19–34. [Google Scholar]
- Leonarduzzi, G.; Sevanian, A.; Sottero, B.; Arkan, M.C.; Biasi, F.; Chiarpotto, E.; Basaga, H.; Poli, G. Up-regulation of the fibrogenic cytokine TGF-β1 by oxysterols: A mechanistic link between cholesterol and atherosclerosis. FASEB J 2001, 15, 1619–1621. [Google Scholar]
- Leonarduzzi, G.; Gamba, P.; Sottero, B.; Kadl, A.; Robbesyn, F.; Calogero, R.A.; Biasi, F.; Chiarpotto, E.; Leitinger, N.; Sevanian, A.; et al. Oxysterol-induced up-regulation of MCP-1 expression and synthesis in macrophage cells. Free Radic. Biol. Med 2005, 39, 1152–1161. [Google Scholar]
- Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Sottero, B.; Kadl, A.; Biasi, F.; Chiarpotto, E.; Leitinger, N.; Vendemiale, G.; Serviddio, G.; et al. Oxidation as a crucial reaction for cholesterol to induce tissue degeneration: CD36 overexpression in human promonocytic cells treated with a biologically relevant oxysterol mixture. Aging Cell 2008, 7, 375–382. [Google Scholar]
- Dzeletovic, S.; Breuer, O.; Lund, E.; Diczfalusy, U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem 1995, 225, 73–80. [Google Scholar]
- Chang, Y.H.; Abdalla, D.S.; Sevanian, A. Characterization of cholesterol oxidation products formed by oxidative modification of low density lipoprotein. Free Radic. Biol. Med 1997, 23, 202–214. [Google Scholar]
- Leonarduzzi, G.; Poli, G.; Sottero, B.; Biasi, F. Activation of the mitochondrial pathway of apoptosis by oxysterols. Front. Biosci 2007, 12, 791–799. [Google Scholar]
- Leonarduzzi, G.; Gargiulo, S.; Gamba, P.; Perrelli, M.G.; Castellano, I.; Sapino, A.; Sottero, B.; Poli, G. Molecular signaling operated by a diet-compatible mixture of oxysterols in up-regulating CD36 receptor in CD68 positive cells. Mol. Nutr. Food Res 2010, 54, S31–S41. [Google Scholar]
- Prunet, C.; Montange, T.; Véjux, A.; Laubriet, A.; Rohmer, J.F.; Riedinger, J.M.; Athias, A.; Lemaire-Ewing, S.; Néel, D.; Petit, J.M.; et al. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry A 2006, 69, 359–373. [Google Scholar]
- Lemaire-Ewing, S.; Berthier, A.; Royer, M.C.; Logette, E.; Corcos, L.; Bouchot, A.; Monier, S.; Prunet, C.; Raveneau, M.; Rébé, C.; et al. 7beta-Hydroxycholesterol and 25-hydroxycholesterol-induced interleukin-8 secretion involves a calcium-dependent activation of c-fos via the ERK1/2 signaling pathway in THP-1 cells: Oxysterols-induced IL-8 secretion is calcium-dependent. Cell. Biol. Toxicol 2009, 25, 127–139. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gargiulo, S.; Gamba, P.; Testa, G.; Sottero, B.; Maina, M.; Guina, T.; Biasi, F.; Poli, G.; Leonarduzzi, G. Molecular Signaling Involved in Oxysterol-Induced β1-Integrin Over-Expression in Human Macrophages. Int. J. Mol. Sci. 2012, 13, 14278-14293. https://doi.org/10.3390/ijms131114278
Gargiulo S, Gamba P, Testa G, Sottero B, Maina M, Guina T, Biasi F, Poli G, Leonarduzzi G. Molecular Signaling Involved in Oxysterol-Induced β1-Integrin Over-Expression in Human Macrophages. International Journal of Molecular Sciences. 2012; 13(11):14278-14293. https://doi.org/10.3390/ijms131114278
Chicago/Turabian StyleGargiulo, Simona, Paola Gamba, Gabriella Testa, Barbara Sottero, Marco Maina, Tina Guina, Fiorella Biasi, Giuseppe Poli, and Gabriella Leonarduzzi. 2012. "Molecular Signaling Involved in Oxysterol-Induced β1-Integrin Over-Expression in Human Macrophages" International Journal of Molecular Sciences 13, no. 11: 14278-14293. https://doi.org/10.3390/ijms131114278




