Content and Color Stability of Anthocyanins Isolated from Schisandra chinensis Fruit
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction pH
2.2. Optimization of Extraction Parameters by Box-Behnken Design (BBD)
2.3. Content and Color Stability of S. chinensis Fruit Anthocyanins
2.3.1. Effect of Heating on Content and Color Stability of Anthocyanins
2.3.2. Effect of Ultrasound on Content and Color Stability of Anthocyanins
2.3.3. Effect of Microwave on Content and Color Stability of Anthocyanins
2.3.4. Effect of Ultraviolet Irradiation on Content and Color Stability of Anthocyanins
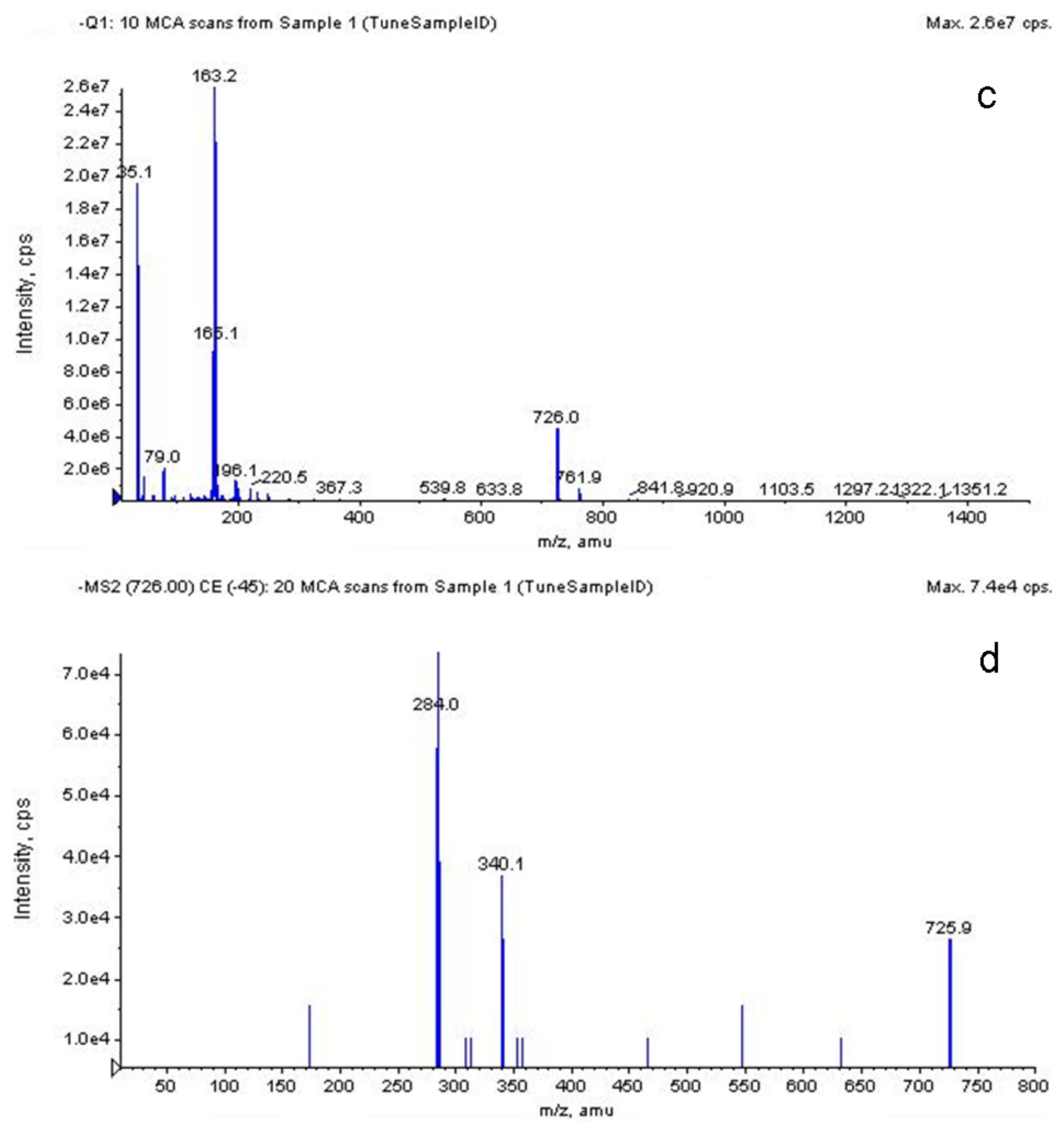
2.3.5. LC-MS Analysis of S. chinensis Major Anthocyanins
3. Experimental Section
3.1. Material
3.2. Methods
3.2.1. The Extract Samples Preparation
3.2.2. Total Monomeric Anthocyanins (TMA)
3.2.3. Colorimetric Study
3.2.4. LC-UV-ESI-MS Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Pharmacopoeia of the People’s Republic of China; Peoples Medicinal Publishing House: Beijing, China, 2010; pp. 61–62.
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar]
- Li, X.N.; Cui, H.; Song, Y.Q.; Liang, Y.Z.; Chau, F.T. Analysis of volatile fractions of Schisandra chinensis (Turcz.) Baill. using GC-MS and chemometric resolution. Phytochem. Anal 2003, 14, 23–33. [Google Scholar]
- Ma, C.H.; Liu, T.T.; Yang, L.; Zu, Y.G.; Chen, X.Q.; Zhang, L.; Zhang, Y.; Zhao, C.J. Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Chromatogr. A 2011, 1218, 8573–8580. [Google Scholar]
- Choi, Y.W.; Takamatsu, S.; Khan, S.I.; Srinivas, P.V.; Ferreira, D.; Zhao, J.; Khan, I.A. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: Structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J. Nat. Prod 2006, 69, 356–359. [Google Scholar]
- Ma, C.H.; Liu, T.T.; Yang, L.; Zu, Y.G.; Yang, F.J.; Zhao, C.J.; Zhang, L.; Zhang, Z.H. Preparation of high purity biphenyl cyclooctene lignans from Schisandra extract by ion exchange resin catalytic transformation combined with macroporous resin separation. J. Chromatogr. B 2011, 879, 3444–3451. [Google Scholar]
- Ma, C.H.; Liu, T.T.; Yang, L.; Zu, Y.G.; Wang, S.Y.; Zhang, R.R. Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal. Chim. Acta 2011, 689, 110–116. [Google Scholar]
- Garzón, G.A.; Wrolstad, R.E. Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chem 2009, 114, 44–49. [Google Scholar]
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Characterization of anthocyanins in Kenyan teas: Extraction and identification. Food Chem 2012, 131, 31–38. [Google Scholar]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem 2012, 130, 9–19. [Google Scholar]
- Lule, S.U.; Xia, W. Food phenolics, pros and cons: A review. Food Rev. Int 2005, 21, 367–388. [Google Scholar]
- Nichenametla, S.N.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci 2006, 46, 161–183. [Google Scholar]
- Lohachoompol, V.; Mulholland, M.; Srzednicki, G.; Craske, J. Determination of anthocyanins in various cultivars of highbush and rabbiteye blueberries. Food Chem 2008, 111, 249–254. [Google Scholar]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic 2009, 119, 275–279. [Google Scholar]
- Bordonaba, J.G.; Crespo, P.; Terry, L.A. A new acetonitrile-free mobile phase for HPLC-DAD determination of individual anthocyanins in blackcurrant and strawberry fruits: A comparison and validation study. Food Chem 2011, 129, 1265–1273. [Google Scholar]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res. Int 2010, 43, 1464–1469. [Google Scholar]
- Elisia, I.; Hu, C.; Popovich, D.G.; Kitts, D.D. Antioxidant assessment of an anthocyanin-enriched blackberry extract. Food Chem 2007, 101, 1052–1058. [Google Scholar]
- Kırca, A.; Özkan, M.; Cemeroğlu, B. Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chem 2007, 101, 212–218. [Google Scholar]
- Zou, T.B.; Wang, M.; Gan, R.Y.; Ling, W.H. Optimization of ultrasound-assisted extraction of anthocyanins from mulberry, using response surface methodology. Int. J. Mol. Sci 2011, 12, 3006–3017. [Google Scholar]
- Liang, Z.C.; Wu, B.H.; Fan, P.G.; Yang, C.X.; Duan, W.; Zheng, X.B.; Liu, C.Y.; Li, S.H. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem 2008, 111, 837–844. [Google Scholar]
- Fan, G.J.; Han, Y.B.; Gu, Z.X.; Gu, F.R. Composition and color stability of anthocyanins extracted from fermented purple sweet potato culture. LWT-Food Sci. Technol 2008, 41, 1412–1416. [Google Scholar]
- Chen, F.; Sun, Y.Z.; Zhao, G.H.; Liao, X.J.; Hu, X.S.; Wu, J.H.; Wang, Z.F. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography-mass spectrometry. Ultrason. Sonochem 2007, 14, 767–778. [Google Scholar]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs. Food Res. Int 2009, 42, 1331–1336. [Google Scholar]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Almeida, D.P.F. Effect of post-harvest practices on flavonoid content of red and white onion cultivars. Food Control 2010, 21, 878–884. [Google Scholar]
- Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal 2010, 23, 592–598. [Google Scholar]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Almeida, D.P.F. Effect of meteorological conditions on antioxidant flavonoids in Portuguese cultivars of white and red onions. Food Chem 2011, 124, 303–308. [Google Scholar]
- Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J. Flavonoids changes in fresh-cut onions during storage in different packaging systems. Food Chem 2011, 124, 652–658. [Google Scholar]
- Pérez-Gregorio, M.R.; Regueiro, J.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 2011, 22, 1108–1113. [Google Scholar]
- García, A.A.; Grande, B.C.; Gándara, J.S. Development of a rapid method based on solid-phase extraction and liquid chromatography with ultraviolet absorbance detection for the determination of polyphenols in alcohol-free beers. J. Chromatogr. A 2004, 1054, 175–180. [Google Scholar]
- Pérez-Gregorio, M.R.; Regueiro, J.; Alonso-González, E.; Pastrana-Castro, L.M.; Simal-Gándara, J. Influence of alcoholic fermentation process on antioxidant activity and phenolic levels from mulberries (Morus nigra L.). LWT-Food Sci. Technol 2011, 44, 1793–1801. [Google Scholar]
- Pérez-Lamela, C.; García-Falcón, M.S.; Simal-Gándara, J.; Orriols-Fernández, I. Influence of grape variety, vine system and enological treatments on the color stability of young red wines. Food Chem 2007, 101, 601–606. [Google Scholar]
- Pérez-Gregorio, M.R.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Comparison of sanitizing technologies on the quality appearance and antioxidant levels in onion slices. Food Control 2011, 22, 2052–2058. [Google Scholar]
- Sun, J.; Yao, J.Y.; Huang, S.X.; Long, X.; Wang, J.B.; Garcia-Garcia, E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) A.C. Smith. Food Chem 2009, 117, 276–281. [Google Scholar]
- Maskan, M. Production of pomegranate (Punica granatum L.) juice concentrate by various heating methods: Color degradation and kinetics. J. Food Eng 2006, 72, 218–224. [Google Scholar]
- Zhang, Y.; Hu, X.S.; Chen, F.; Wu, J.H.; Liao, X.J.; Wang, Z.F. Stability and color characteristics of PEF-treated cyanidin-3-glucoside during storage. Food Chem 2008, 106, 669–676. [Google Scholar]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol 2008, 9, 161–169. [Google Scholar]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol 2004, 15, 261–266. [Google Scholar]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Cullen, P.J. Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. J. Agric. Food Chem 2008, 56, 10071–10077. [Google Scholar]
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of sonication on retention of anthocyanins in blackberry juice. J. Food Eng 2009, 93, 166–171. [Google Scholar]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem 2010, 17, 598–604. [Google Scholar]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem 2011, 124, 1238–1243. [Google Scholar]
- Yang, Z.D.; Zhai, W.W. Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC-MS. Innov. Food Sci. Emerg. Technol 2010, 11, 470–476. [Google Scholar]
- Golmakani, M.T.; Rezaei, K. Comparison of microwave-assisted hydrodistillation with the traditional hydrodistillation method in the extraction of essential oils from Thymus vulgaris L. Food Chem 2008, 109, 925–930. [Google Scholar]
- Patil, S.; Bourke, P.; Frias, J.M.; Tiwari, B.K.; Cullen, P.J. Inactivation of Escherichia coli in orange juice using ozone. Innov. Food Sci. Emerg. Technol 2009, 10, 551–557. [Google Scholar]
- Pala, Ç.U.; Toklucu, A.K. Effect of UV-C light on anthocyanin content and other quality parameters of pomegranate juice. J. Food Compos. Anal. 2011, 24, 790–795. [Google Scholar]
- Mar, P.H.; Poppi, R.J.; Scarminio, I.S.; Tauler, R. Investigation of the pH effect and UV radiation on kinetic degradation of anthocyanin mixtures extracted from Hibiscus acetosella. Food Chem 2011, 125, 1020–1027. [Google Scholar]
- Lee, S.S.; Lee, E.M.; An, B.C.; Kim, T.H.; Lee, K.S.; Cho, J.Y.; Yoo, S.H.; Bae, J.S.; Chung, B.Y. Effects of irradiation on decolourisation and biological activity in Schizandra chinensis extracts. Food Chem 2011, 125, 214–220. [Google Scholar]
- Kim, S.H.; Lee, B.H.; Kim, J.C.; Choi, S.S.; Kim, G.W.; Joo, M.H.; Yoo, S.H. Compositional characterization and colorant identification of Omija (Schizandra chinensis) fruit extract. Food Sci. Biotechnol 2008, 17, 787–793. [Google Scholar]
- Goiffon, J.P.; Mouly, P.P.; Gaydou, E.M. Anthocyanin pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar]
- Kim, S.H.; Joo, M.H.; Yoo, S.H. Structural identification and antioxidant properties of major anthocyanin extracted from Omija (Schizandra chinensis) fruit. J. Food Sci 2009, 74, 134–140. [Google Scholar]
- Hurtado, N.H.; Morales, A.L.; González-Miret, M.L.; Escudero-Gilete, M.L.; Heredia, F.J. Colour, pH stability and antioxidant activity of anthocyanin rutinosides isolated from tamarillo fruit (Solanum betaceum Cav.). Food Chem 2009, 117, 88–93. [Google Scholar]
- Choi, M.H.; Kim, G.H.; Lee, H.S. Effect of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res. Int 2002, 35, 753–759. [Google Scholar]

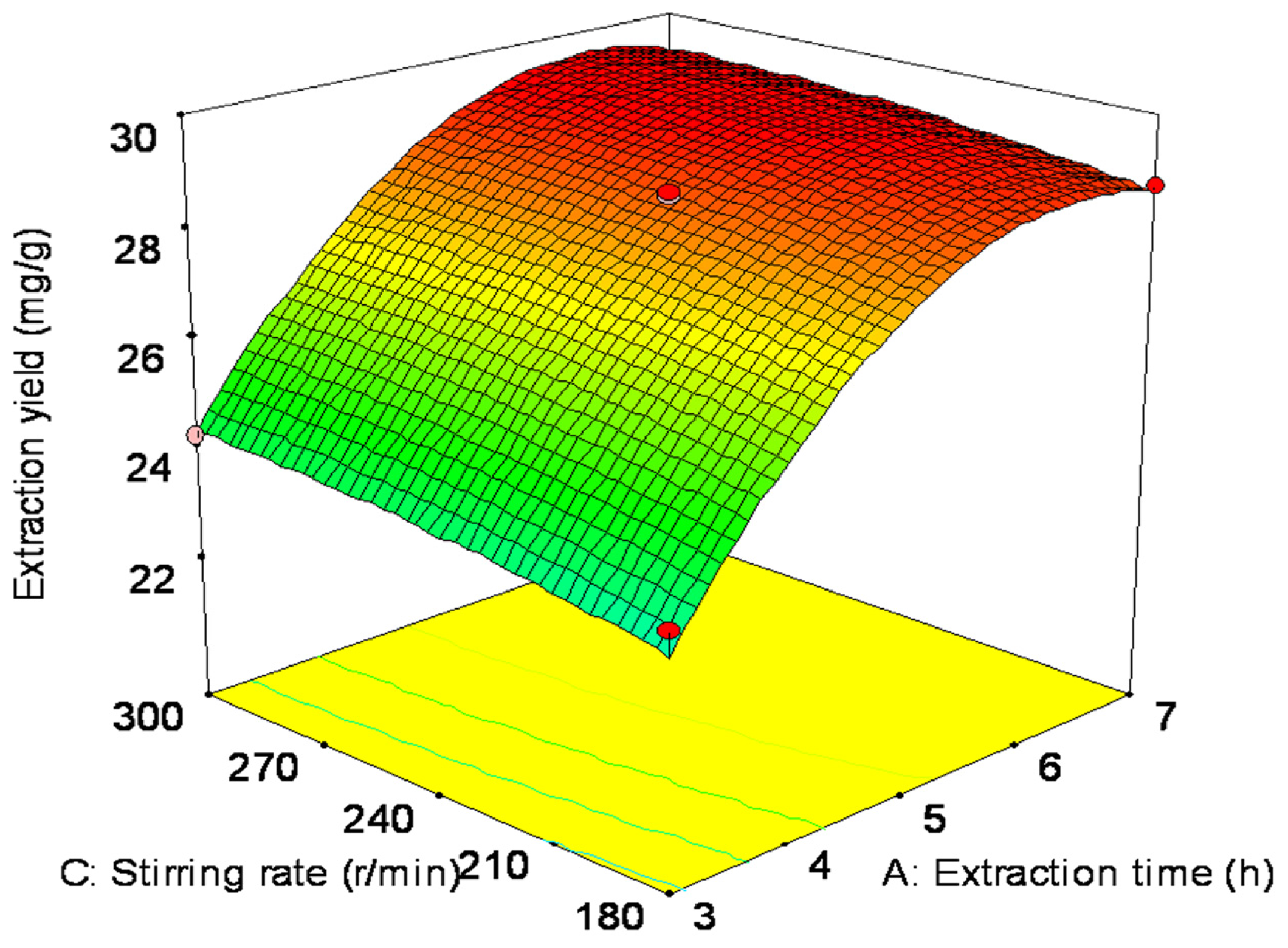
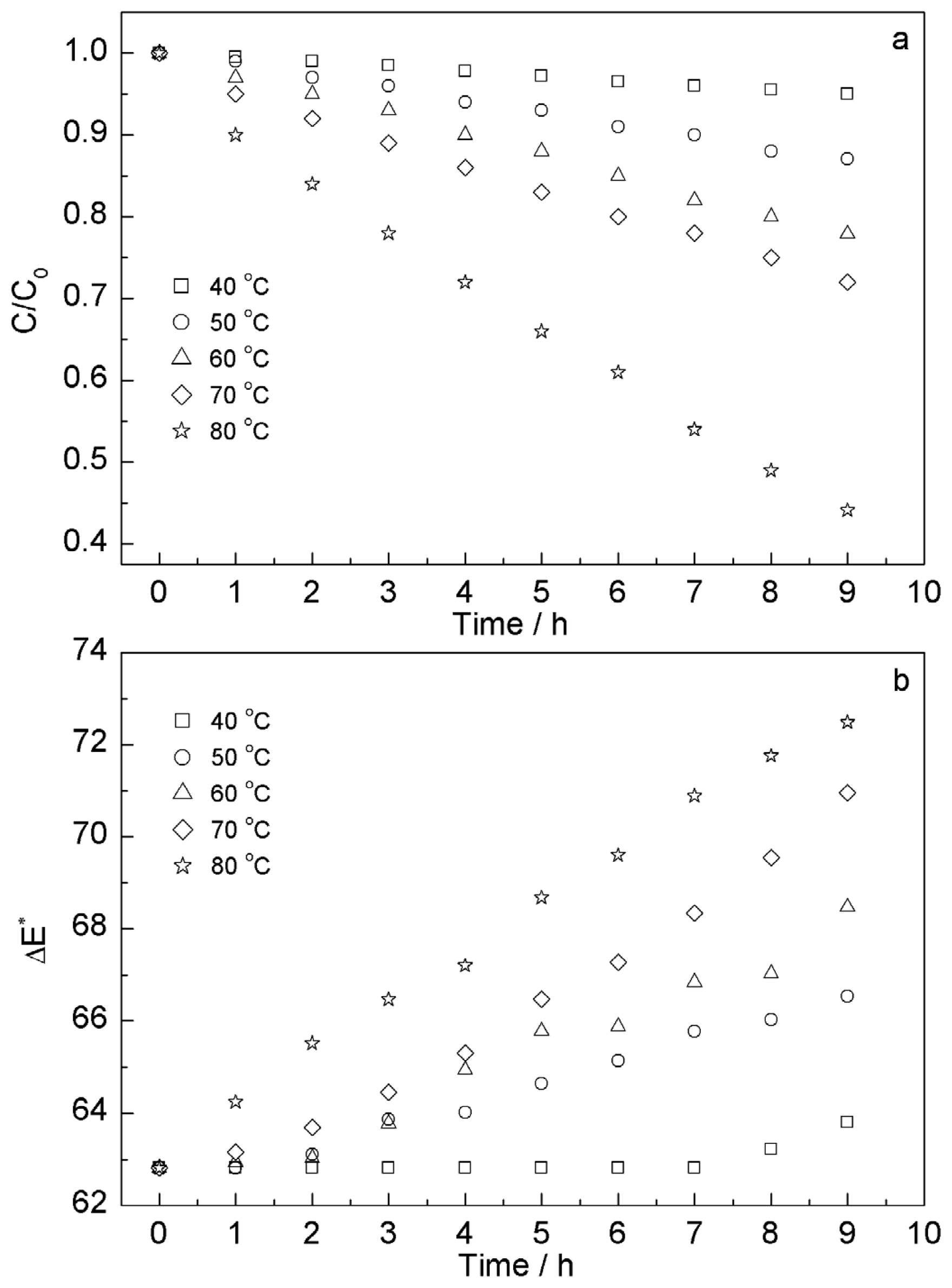
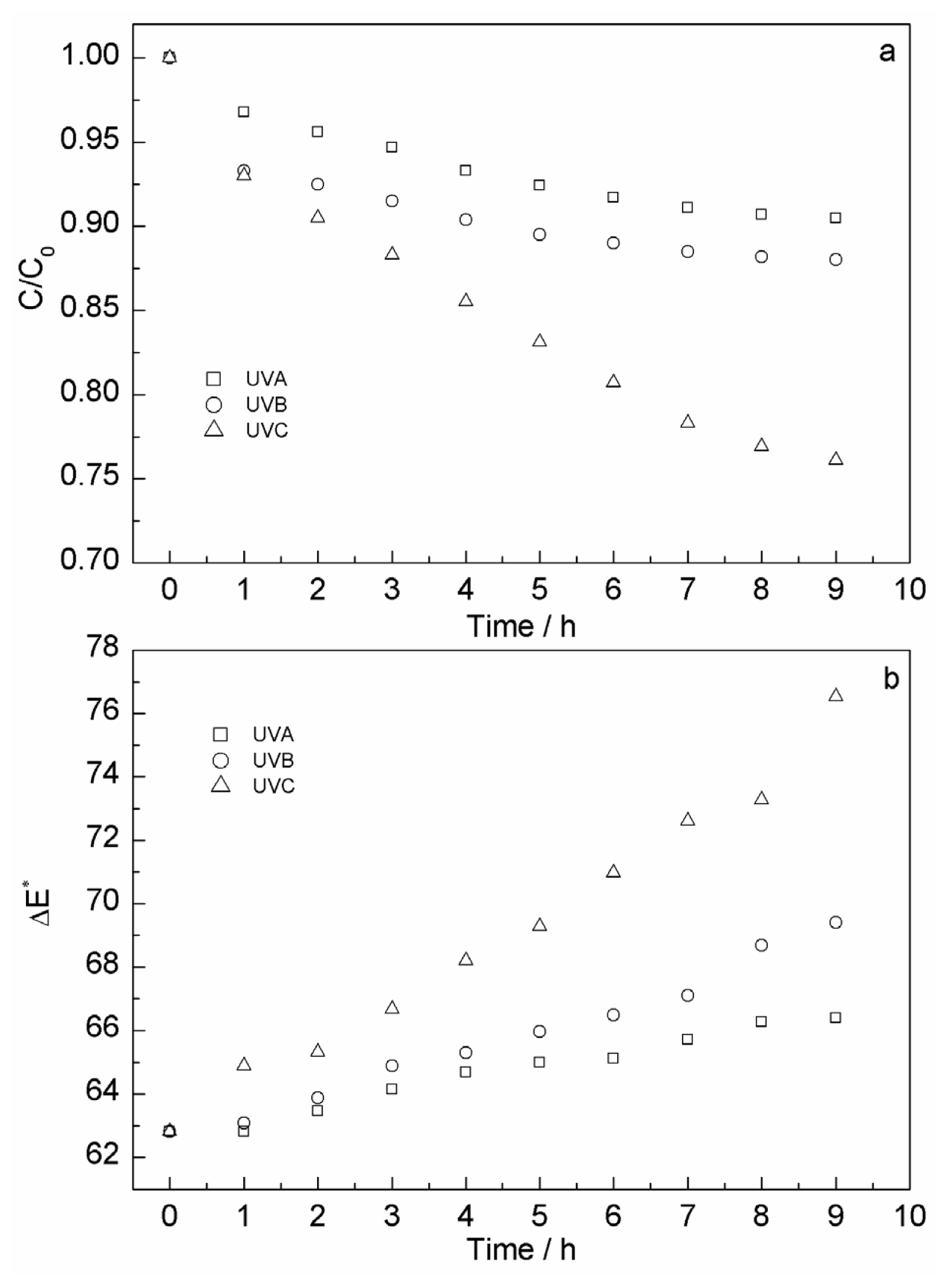
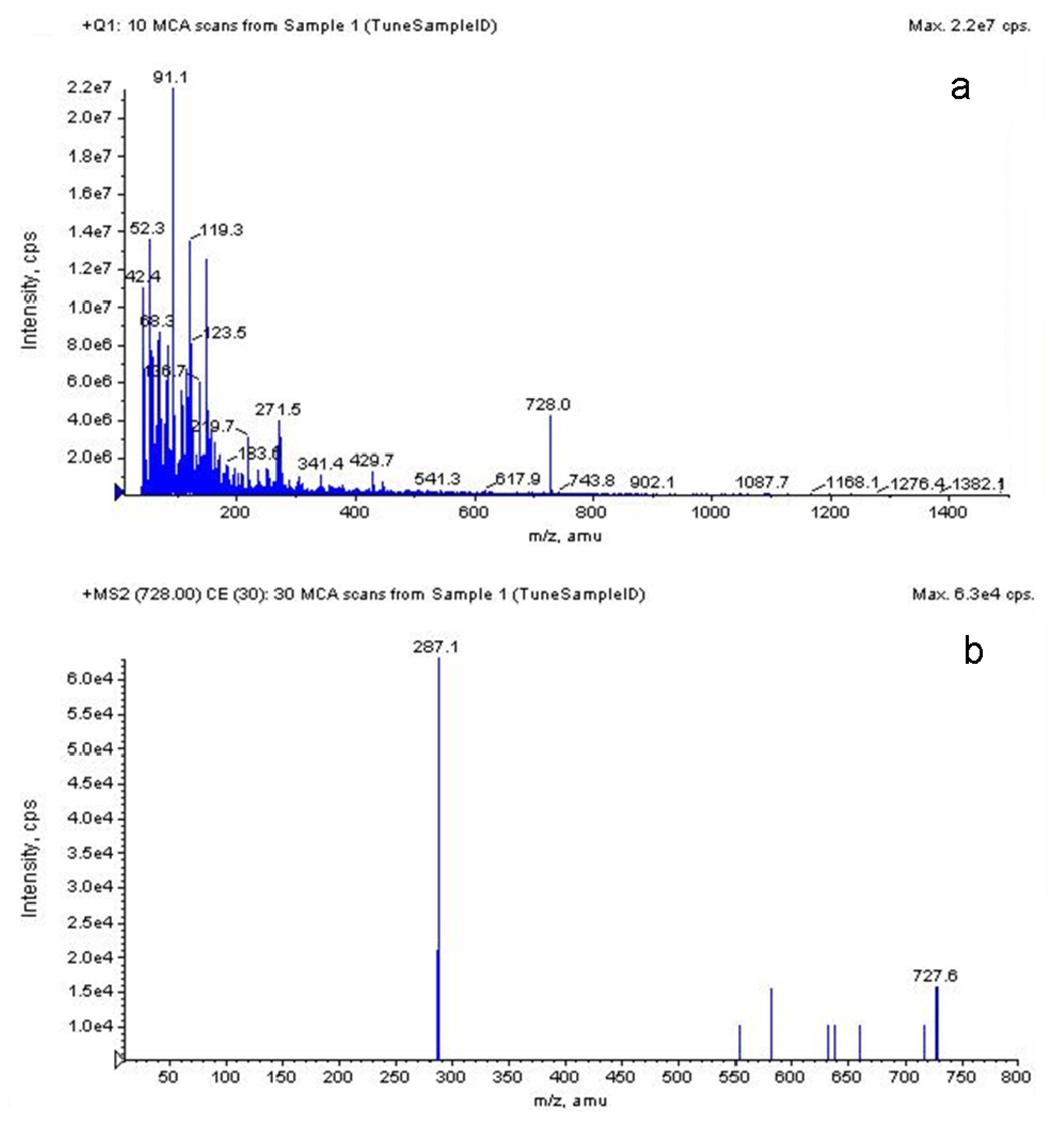
| Run | Factor A | Factor B | Factor C | Response |
|---|---|---|---|---|
| Extraction time (h) | Solid-liquid ratio (g/mL) | Stirring rate (r/min) | Extraction yield (mg/g) | |
| 1 | 3.0 | 1:20 | 240 | 26.43 |
| 2 | 3.0 | 1:15 | 180 | 23.86 |
| 3 | 3.0 | 1:10 | 240 | 20.37 |
| 4 | 5.0 | 1:20 | 300 | 29.13 |
| 5 | 5.0 | 1:15 | 240 | 28.61 |
| 6 | 7.0 | 1:10 | 240 | 28.33 |
| 7 | 7.0 | 1:15 | 300 | 28.92 |
| 8 | 7.0 | 1:20 | 240 | 29.30 |
| 9 | 5.0 | 1:15 | 240 | 28.60 |
| 10 | 5.0 | 1:10 | 300 | 27.97 |
| 11 | 5.0 | 1:20 | 180 | 28.84 |
| 12 | 5.0 | 1:10 | 180 | 25.66 |
| 13 | 5.0 | 1:15 | 240 | 28.60 |
| 14 | 5.0 | 1:15 | 240 | 28.67 |
| 15 | 5.0 | 1:15 | 240 | 28.65 |
| 16 | 3.0 | 1:15 | 300 | 24.25 |
| 17 | 7.0 | 1:15 | 180 | 28.77 |
| Source | Sum of squares | Df | Mean square | F-value | p-Value |
|---|---|---|---|---|---|
| Model b | 95.74 | 9 | 10.64 | 45.56 | <0.0001 |
| A | 52.07 | 1 | 52.07 | 223.02 | <0.0001 |
| B | 16.16 | 1 | 16.16 | 69.21 | <0.0001 |
| C | 1.23 | 1 | 1.23 | 5.28 | 0.0552 |
| AB | 6.48 | 1 | 6.48 | 27.74 | 0.0012 |
| AC | 0.014 | 1 | 0.014 | 0.062 | 0.8110 |
| BC | 1.02 | 1 | 1.02 | 4.37 | 0.0749 |
| A2 | 16.58 | 1 | 16.58 | 71.00 | <0.0001 |
| B2 | 1.20 | 1 | 1.02 | 5.15 | 0.0576 |
| C2 | 0.15 | 1 | 0.15 | 0.66 | 0.4423 |
| Linear | 27.91 | 9 | 3.10 | 3010.56 | <0.0001 |
| 2FI | 20.40 | 6 | 3.40 | 3300.38 | <0.0001 |
| Quadratic | 1.63 | 3 | 0.54 | 527.60 | <0.0001 |
| Cubic | 0.00 | 0 | - | - | - |
| Residual | 1.63 | 7 | 0.23 | - | - |
| Lack of fit | 1.63 | 3 | 0.54 | 527.60 | <0.0001 |
| Pure Error | 4.12 × 10−3 | 4 | 1.03 × 10−3 | - | - |
| Cor Total | 97.38 | 16 | - | - | - |
| Index marka | Values |
|---|---|
| Std. Dev. | 0.48 |
| Mean | 27.35 |
| C.V. % | 1.77 |
| PRESS | 26.09 |
| R-Squared | 0.9832 |
| Adjust R-Squared | 0.9616 |
| Predicted R-Squared | 0.7321 |
| Adequacy Precision | 23.128 |
| Temperature (°C) | First-order kinetic equation | Kinetic constant (k) | Correlation coefficient (r) |
|---|---|---|---|
| 40 | Y = − 0.0057 X + 1.0066 | 0.0057 | 0.9988 |
| 50 | Y = − 0.0148 X + 1.0165 | 0.0148 | 0.9982 |
| 60 | Y = − 0.0248 X + 1.0242 | 0.0248 | 0.9989 |
| 70 | Y = − 0.0298 X + 1.0140 | 0.0298 | 0.9969 |
| 80 | Y = − 0.0604 X + 1.0305 | 0.0604 | 0.9974 |
| Treat time of ultrasonication (min) | C/C0a ± SD b (%) | ΔE* c ± SD b |
|---|---|---|
| 0 | 1.000 | 62.81 |
| 10 | 98.0 ± 0.7 | 65.17 ± 2.5 |
| 20 | 97.0 ± 0.7 | 65.82 ± 2.6 |
| 30 | 96.0 ± 0.5 | 66.35 ± 1.7 |
| 40 | 96.0 ± 0.4 | 66.45 ± 2.5 |
| 50 | 96.0 ± 0.7 | 66.54 ± 2.4 |
| 60 | 95.0 ± 0.6 | 66.95 ± 1.7 |
| 70 | 94.0 ± 0.6 | 67.38 ± 2.4 |
| 80 | 94.0 ± 0.6 | 67.86 ± 2.6 |
| 90 | 93.0 ± 0.4 | 67.88 ± 2.7 |
| Treat time of microwave (min) | C/C0a± SDb(%) | ΔE* c± SDb |
| 0 | 1.000 | 62.81 |
| 5 | 95.0 ± 0.6 | 67.79 ± 2.6 |
| 10 | 94.0 ± 0.7 | 67.92 ± 2.4 |
| 15 | 93.0 ± 0.8 | 68.57 ± 2.7 |
| 20 | 93.0 ± 0.4 | 68.90 ± 2.7 |
| 25 | 92.0 ± 0.4 | 69.07 ± 2.4 |
| 30 | 91.0 ± 0.5 | 69.26 ± 2.4 |
| 35 | 91.0 ± 0.6 | 69.86 ± 1.7 |
| 40 | 90.0 ± 0.7 | 70.49 ± 1.5 |
| 45 | 90.0 ± 0.8 | 70.65 ± 2.5 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, C.; Yang, L.; Yang, F.; Wang, W.; Zhao, C.; Zu, Y. Content and Color Stability of Anthocyanins Isolated from Schisandra chinensis Fruit. Int. J. Mol. Sci. 2012, 13, 14294-14310. https://doi.org/10.3390/ijms131114294
Ma C, Yang L, Yang F, Wang W, Zhao C, Zu Y. Content and Color Stability of Anthocyanins Isolated from Schisandra chinensis Fruit. International Journal of Molecular Sciences. 2012; 13(11):14294-14310. https://doi.org/10.3390/ijms131114294
Chicago/Turabian StyleMa, Chunhui, Lei Yang, Fengjian Yang, Wenjie Wang, Chunjian Zhao, and Yuangang Zu. 2012. "Content and Color Stability of Anthocyanins Isolated from Schisandra chinensis Fruit" International Journal of Molecular Sciences 13, no. 11: 14294-14310. https://doi.org/10.3390/ijms131114294





