Selection of Suitable Reference Genes for Normalization of Quantitative Real-Time PCR in Cartilage Tissue Injury and Repair in Rabbits
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
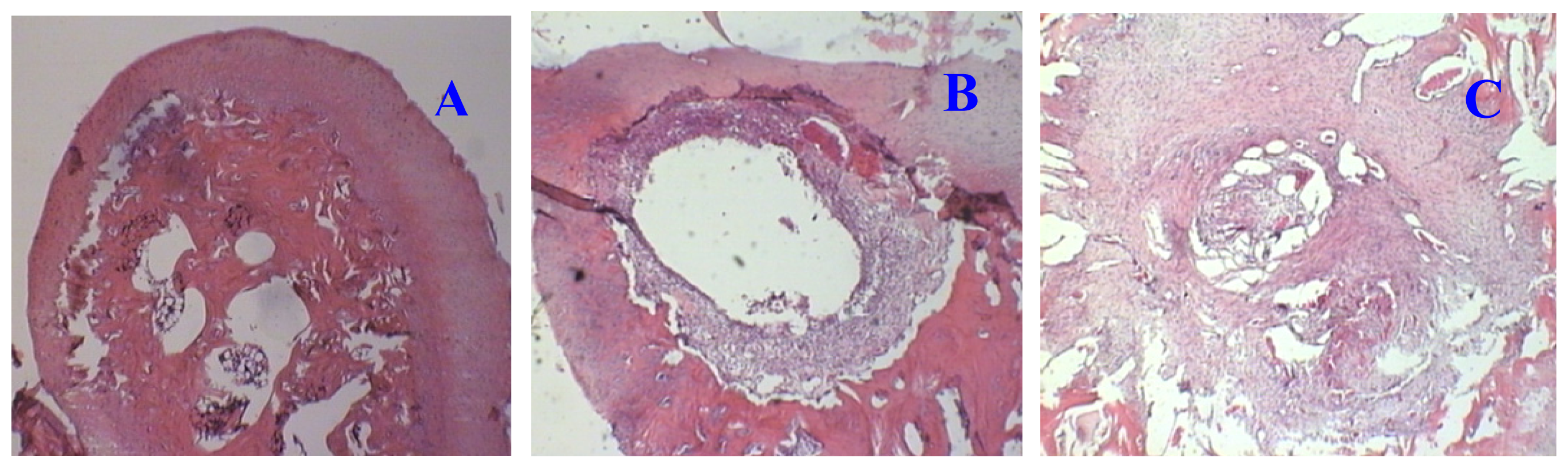
2.1.1. Hematoxylin and Eosin (H and E) Staining
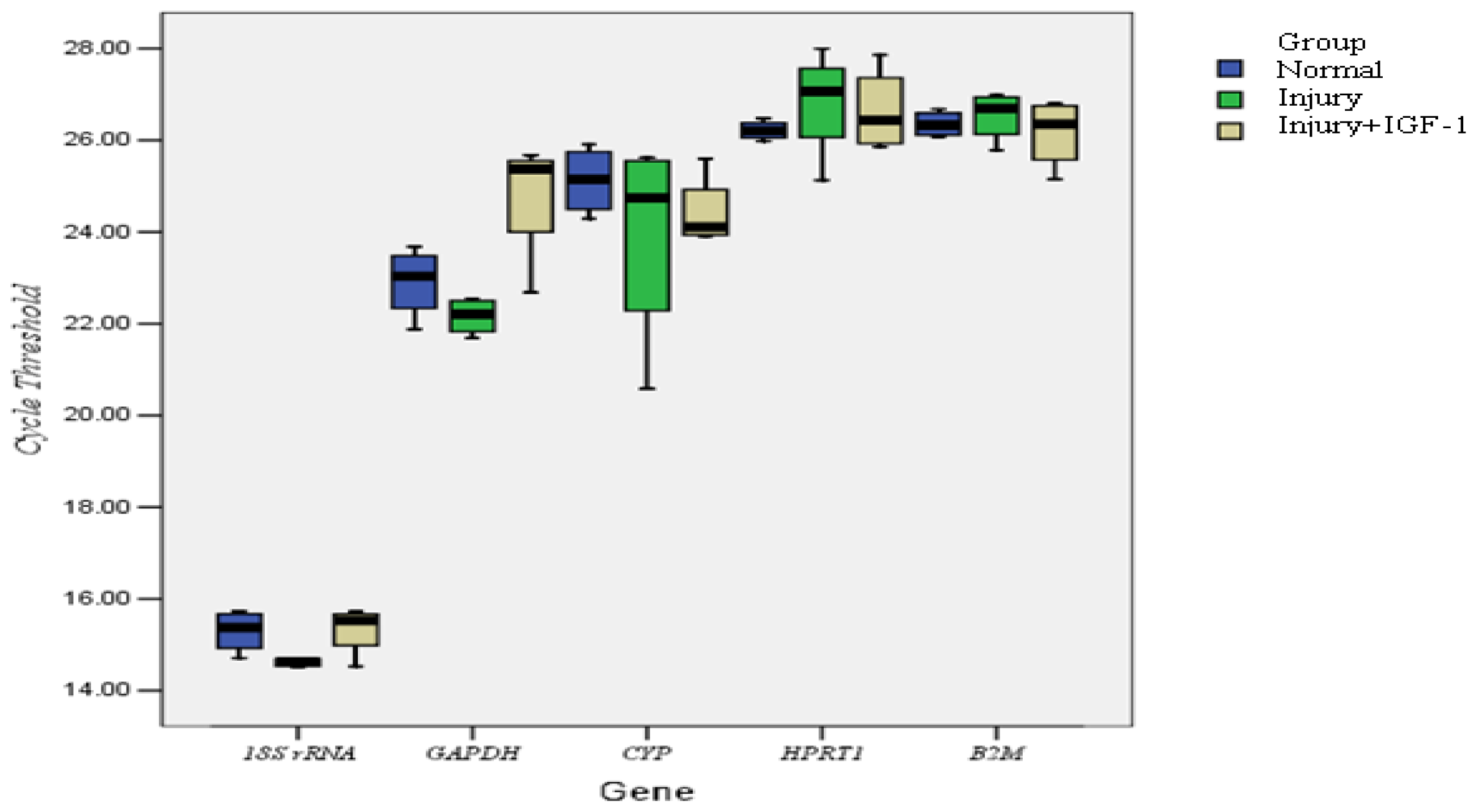
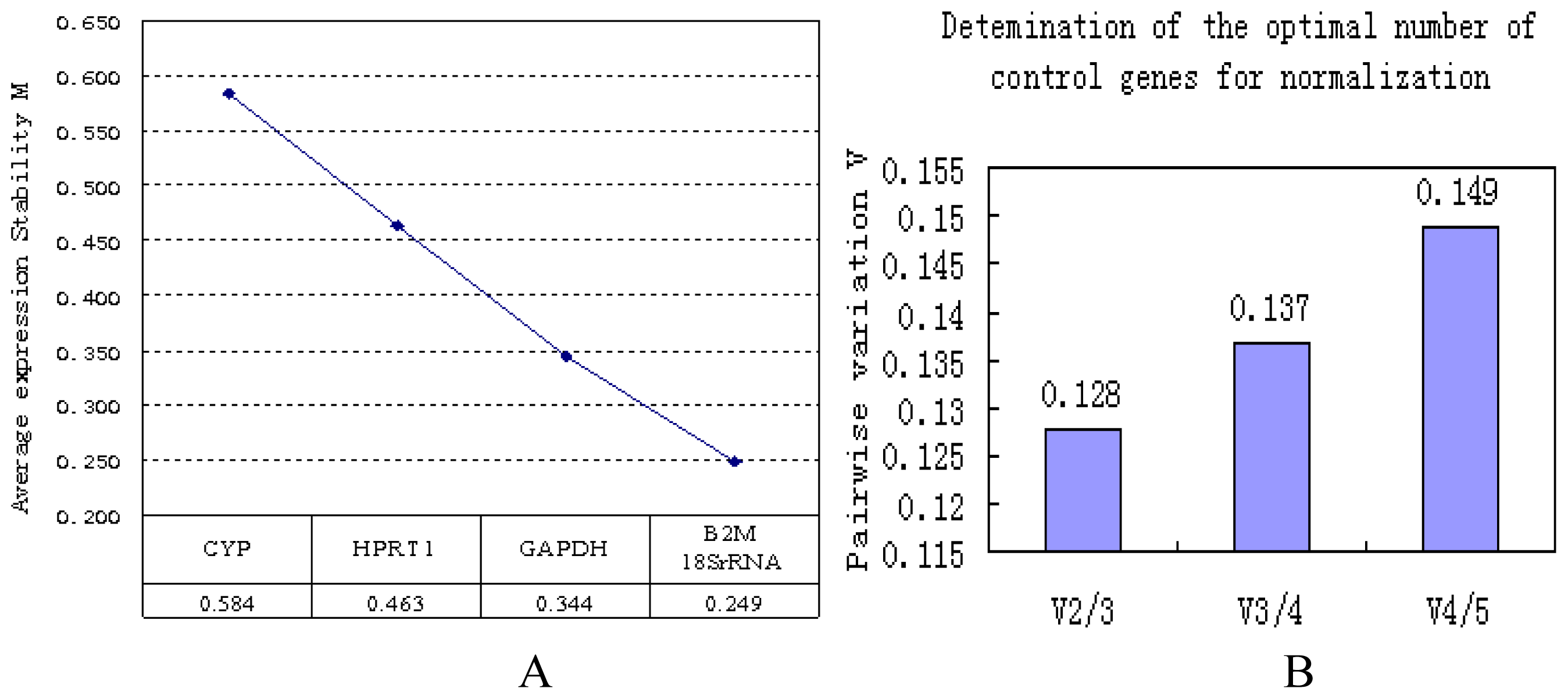
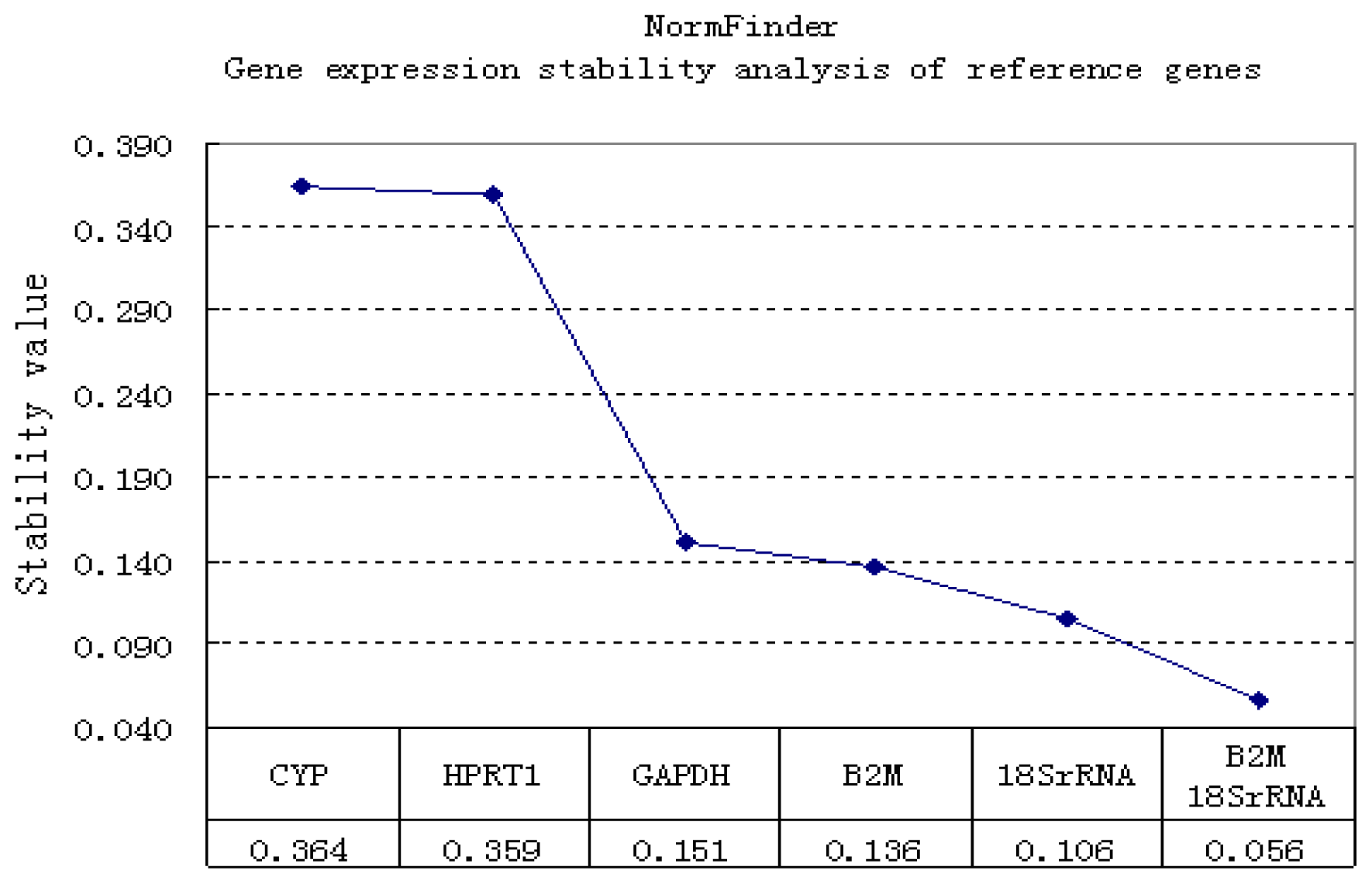
2.1.2. Correlation of Reference Genes
2.2. Discussion
3. Experimental Section
3.1. Materials
3.2. Injury to Articular Cartilage
3.3. Histological Evaluation of Articular Cartilage Injury and Repair
3.4. RNA Isolation and RT-qPCR Amplification of Reference
3.5. Expression Data Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar]
- Bustin, S.A. Why the need for qPCR publication guidelines?—The case for MIQE. Methods 2010, 50, 217–226. [Google Scholar]
- Bustin, S.A.; Nolan, T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J. Biomol. Tech 2004, 15, 155–166. [Google Scholar]
- Imbeaud, S.; Graudens, E.; Boulanger, V.; Barlet, X.; Zaborski, P.; Eveno, E.; Mueller, O.; Schroeder, A.; Auffray, C. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Res 2005, 33, e56. [Google Scholar]
- Li, Y.L.; Ye, F.; Hu, Y.; Lu, W.G.; Xie, X. Identification of suitable reference genes for gene expression studies of human serous ovarian cancer by real-time polymerase chain reaction. Anal. Biochem 2009, 394, 110–116. [Google Scholar]
- Kowalewska, M.; Danska-Bidzinska, A.; Bakula-Zalewska, E.; Bidzinski, M. Identification of suitable reference genes for gene expression measurement in uterine sarcoma and carcinosarcoma tumors. Clin. Biochem 2012, 45, 368–371. [Google Scholar]
- Cinar, M.U.; Islam, M.A.; Uddin, M.J.; Tholen, E.; Tesfaye, D.; Looft, C.; Schellander, K. Evaluation of suitable reference genes for gene expression studies in porcine alveolar macrophages in response to LPS and LTA. BMC Res. Notes 2012, 5, 107. [Google Scholar]
- Uddin, M.J.; Cinar, M.U.; Tesfaye, D.; Looft, C.; Tholen, E.; Schellander, K. Age-related changes in relative expression stability of commonly used housekeeping genes in selected porcine tissues. BMC Res. Notes 2011, 4, 441. [Google Scholar]
- Nelissen, K.; Smeets, K.; Mulder, M.; Hendriks, J.J.; Ameloot, M. Selection of reference genes for gene expression studies in rat oligodendrocytes using quantitative real time PCR. J. Neurosci. Methods 2010, 187, 78–83. [Google Scholar]
- Wang, F.; Wang, J.; Liu, D.; Su, Y. Normalizing genes for real-time polymerase chain reaction in epithelial and nonepithelial cells of mouse small intestine. Anal. Biochem 2010, 399, 211–217. [Google Scholar]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 2005, 6, 279–284. [Google Scholar]
- Ho-Pun-Cheung, A.; Cellier, D.; Lopez-Crapez, E. Considerations for normalisation of RT-qPCR in oncology (In French). Ann. Biol. Clin 2008, 66, 121–129. [Google Scholar]
- Mamo, S.; Gal, A.B.; Polgar, Z.; Dinnyes, A. Expression profiles of the pluripotency marker gene POU5F1 and validation of reference genes in rabbit oocytes and preimplantation stage embryos. BMC Mol. Biol 2008, 9, 67. [Google Scholar]
- Seol, D.; Choe, H.; Zheng, H.; Jang, K.; Ramakrishnan, P.S.; Lim, T.H.; Martin, J.A. Selection of reference genes for normalization of quantitative real-time PCR in organ culture of the rat and rabbit intervertebral disc. BMC Res. Notes 2011, 4, 162. [Google Scholar]
- Pombo-Suarez, M.; Calaza, M.; Gomez-Reino, J.J.; Gonzalez, A. Reference genes for normalization of gene expression studies in human osteoarthritic articular cartilage. BMC Mol. Biol 2008, 9, 17. [Google Scholar]
- Foldager, C.B.; Munir, S.; Ulrik-Vinther, M.; Søballe, K.; Bünger, C.; Lind, M. Validation of suitable house keeping genes for hypoxia-cultured human chondrocytes. BMC Mol. Biol 2009, 10, 94. [Google Scholar]
- Majerowicz, D.; Alves-Bezerra, M.; Logullo, R.; Fonseca-de-Souza, A.L.; Meyer-Fernandes, J.R.; Braz, G.R.; Gondim, K.C. Looking for reference genes for real-time quantitative PCR experiments in Rhodnius prolixus (Hemiptera: Reduviidae). Insect Mol. Biol 2011, 20, 713–722. [Google Scholar]
- Kulkarni, B.; Mohammed, I.; Hopkinson, A.; Dua, H.S. Validation of endogenous control genes for gene expression studies on human ocular surface epithelium. PLoS One 2011, 6, e22301. [Google Scholar]
- Barsalobres-Cavallari, C.F.; Severino, F.E.; Maluf, M.P.; Maia, I.G. Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Mol. Bio 2009, 10, 1. [Google Scholar]
- Paim, R.M.; Pereira, M.H.; di Ponzio, R.; Rodrigues, J.O.; Guarneri, A.A.; Gontijo, N.F.; Araújo, R.N. Validation of reference genes for expression analysis in the salivary gland and the intestine of Rhodnius prolixus (Hemiptera, Reduviidae) under different experimental conditions by quantitative real-time PCR. BMC Res. Notes 2012, 5, 128. [Google Scholar]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002, 3, research0034. [Google Scholar]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 2004, 64, 5245–5250. [Google Scholar]
- Rho, H.W.; Lee, B.C.; Choi, E.S.; Choi, I.J.; Lee, Y.S.; Goh, S.H. Identification of valid reference genes for gene expression studies of human stomach cancer by reverse transcription-qPCR. BMC Cancer 2010, 10, 240. [Google Scholar]
- Ferreira, E.; Cronjé, M.J. Selection of suitable reference genes for quantitative real-time PCR in apoptosis-induced MCF-7 breast cancer cells. Mol. Biotechnol 2012, 50, 121–128. [Google Scholar]
- Peletto, S.; Bertuzzi, S.; Campanella, C.; Modesto, P.; Maniaci, M.G.; Bellino, C.; Ariello, D.; Quasso, A.; Caramelli, M.; Acutis, P.L. Evaluation of internal reference genes for quantitative expression analysis by real-time PCR in ovine whole blood. Int. J. Mol. Sci 2011, 12, 7732–7747. [Google Scholar]
- Chervoneva, I.; Li, Y.; Schulz, S.; Croker, S.; Wilson, C.; Waldman, S.A.; Hyslop, T. Selection of optimal reference genes for normalization in quantitative RT-PCR. BMC Bioinformatics 2010, 11, 253. [Google Scholar]
- Mohelnikova-Duchonova, B.; Oliverius, M.; Honsova, E.; Soucek, P. Evaluation of reference genes and normalization strategy for quantitative real-time PCR in human pancreatic carcinoma. Dis. Markers 2012, 32, 203–210. [Google Scholar]
- Ledderose, C.; Heyn, J.; Limbeck, E.; Kreth, S. Selection of reliable reference genes for quantitative real-time PCR in human T cells and neutrophils. BMC Res. Notes 2011, 4, 427. [Google Scholar]
- Redler, L.H.; Caldwell, J.M.; Schulz, B.M.; Levine, W.N. Management of articular cartilage defects of the knee. Phys. Sportsmed 2012, 40, 20–35. [Google Scholar]
- Balcom, N.T.; Berg-Johansen, B.; Dills, K.J.; van Donk, J.R.; Williams, G.M.; Chen, A.C.; Hazelwood, S.J.; Sah, R.L.; Klisch, S.M. In vitro articular cartilage growth with sequential application of IGF-1 and TGF-β1 enhances volumetric growth and maintains compressive properties. J. Biomech. Eng 2012, 134, 031001. [Google Scholar]
- Ng, K.W.; O’Conor, C.J.; Kugler, L.E.; Cook, J.L.; Ateshian, G.A.; Hung, C.T. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann. Biomed. Eng 2011, 39, 2491–2500. [Google Scholar]
- Williams, G.M.; Dills, K.J.; Flores, C.R.; Stender, M.E.; Stewart, K.M.; Nelson, L.M.; Chen, A.C.; Masuda, K.; Hazelwood, S.J.; Klisch, S.M.; et al. Differential regulation of immature articular cartilage compressive moduli and Poisson’s ratios by in vitro stimulation with IGF-1 and TGF-beta1. J. Biomech 2010, 43, 2501–2507. [Google Scholar]
- Suzuki, S.; Itoh, K.; Ohyama, K. Local administration of IGF-I stimulates the growth of mandibular condyle in mature rats. Am. J. Orthod 2004, 31, 138–143. [Google Scholar]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil 2006, 14, 403–412. [Google Scholar]
- Penna, I.; Vella, S.; Gigoni, A.; Russo, C.; Cancedda, R.; Pagano, A. Selection of candidate housekeeping genes for normalization in human postmortem brain samples. Int. J. Mol. Sci 2011, 12, 5461–5470. [Google Scholar]
- Bas, A.; Forsberg, G.; Hammarström, S.; Hammarström, M.L. Utility of the housekeeping genes 18S rRNA, beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand. J. Immunol 2004, 59, 566–573. [Google Scholar]
- Tang, R.; Dodd, A.; Lai, D.; McNabb, W.C.; Love, D.R. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin 2007, 39, 384–390. [Google Scholar]
- Kim, I.; Yang, D.; Tang, X.; Carroll, J.L. Reference gene validation for qPCR in rat carotid body during postnatal development. BMC Res. Notes 2011, 4, 440. [Google Scholar]
- Farrokhi, A.; Eslaminejad, M.B.; Nazarian, H.; Moradmand, A.; Samadian, A.; Akhlaghi, A. Appropriate reference gene selection for real-time PCR data normalization during rat mesenchymal stem cell differentiation. Cell Mol. Biol. (Noisy-le-grand) 2012, 58, OL1660–1670. [Google Scholar]
- Stephens, A.S.; Stephens, S.R.; Morrison, N.A. Internal control genes for quantitative RT-PCR expression analysis in mouse osteoblasts, osteoclasts and macrophages. BMC Res. Notes 2011, 4, 410. [Google Scholar]
- Studer, D.; Lischer, S.; Jochum, W.; Ehrbar, M.; Zenobi-Wong, M.; Maniura-Weber, K. Ribosomal protein L13a as a reference gene for human bone marrow-derived mesenchymal stromal cells during expansion, adipo-, chondro-, and osteogenesis. Tissue Eng 2012, 18, 10. [Google Scholar]
- Maccoux, L.J.; Clements, D.N.; Salway, F.; Day, P.J. Identification of new reference genes for the normalisation of canine osteoarthritic joint tissue transcripts from microarray data. BMC Mol. Biol 2007, 8, 62. [Google Scholar]
- Zang, R.; Bai, J.; Xu, H.; Zhang, L.; Yang, J.; Yang, L.; Lu, J.; Wu, J. Selection of suitable reference genes for real-time quantitative PCR studies in Lanzhou fat-tailed sheep (Ovis aries). Asian J. Anim. Vet. Adv 2011, 6, 789–804. [Google Scholar]
- Dundas, J.; Ling, M. Reference genes for measuring mRNA expression. Theory Biosci. 2012. [Google Scholar] [CrossRef]
- Too, I.H.; Ling, M.H. Signal peptidase complex subunit 1 and Hydroxyacyl-CoA dehydrogenase beta subunit are suitable reference genes in human lungs. ISRN Bioinforma. 2012, 2012. [Google Scholar] [CrossRef]
| Gene name | Gene symbol | Accession number | Amplicon length (bp) | Primer sequences |
|---|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH18S rRNA | NM_001082253.1 | 198 | Forward: ATCCATTCATTgACCTCCACTAC |
| Reverse: gTACTgggCACCAgCATCAC | ||||
| 18S Ribosomal RNA | 18S rRNA | NR_033238.1 | 167 | Forward: ATCAgATACCgTCgTAgTTC |
| Reverse: TTCCgTCAATTCCTTTAAg | ||||
| Beta-2-microglobulin | B2M | XM_002717921.1 | 157 | Forward: AACgTggAACAgTCAgACC |
| Reverse: AgTAATCTCgATCCCATTTC | ||||
| Cyclophilin | CYP | ABB45383.1 | 163 | Forward: gggAgAgAgAggATATggATAC |
| Reverse: AATgCCATAgTgCTTCAgC | ||||
| Hypoxanthine phosphoribosyl transferase | HPRT1 | NM_001105671.1 | 175 | Forward: TgATTAgTgATgATgAACCg |
| Reverse: CACACAgAgggCTACAATg | ||||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, X.-X.; Zhao, R.-L.; Song, W.; Chu, H.-R.; Li, M.; Song, S.-Y.; Li, G.-Z.; Liang, D.-C. Selection of Suitable Reference Genes for Normalization of Quantitative Real-Time PCR in Cartilage Tissue Injury and Repair in Rabbits. Int. J. Mol. Sci. 2012, 13, 14344-14355. https://doi.org/10.3390/ijms131114344
Peng X-X, Zhao R-L, Song W, Chu H-R, Li M, Song S-Y, Li G-Z, Liang D-C. Selection of Suitable Reference Genes for Normalization of Quantitative Real-Time PCR in Cartilage Tissue Injury and Repair in Rabbits. International Journal of Molecular Sciences. 2012; 13(11):14344-14355. https://doi.org/10.3390/ijms131114344
Chicago/Turabian StylePeng, Xiao-Xiang, Rong-Lan Zhao, Wei Song, Hai-Rong Chu, Meng Li, Shu-Ya Song, Guang-Zhou Li, and Dong-Chun Liang. 2012. "Selection of Suitable Reference Genes for Normalization of Quantitative Real-Time PCR in Cartilage Tissue Injury and Repair in Rabbits" International Journal of Molecular Sciences 13, no. 11: 14344-14355. https://doi.org/10.3390/ijms131114344




