Non-Alcoholic Fatty Liver Disease Is not Related to the Incidence of Diabetic Nephropathy in Type 2 Diabetes
Abstract
:1. Introduction
2. Results and Discussion
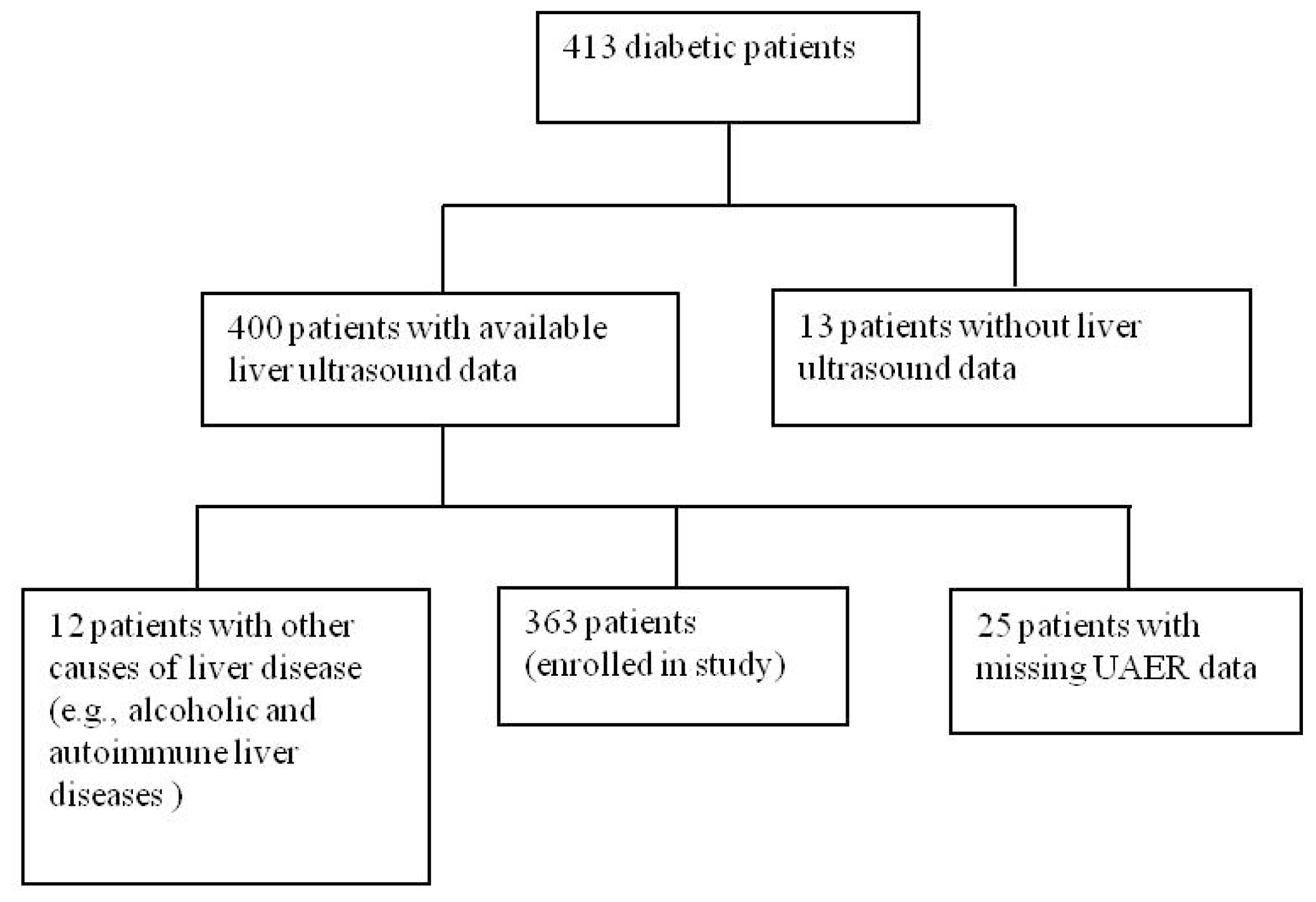
2.1. Study Participants
2.2. The Clinical Features and Biochemical Characteristics of Participants
2.3. Incidence of Diabetic Nephropathy in Participants
2.4. Risk Factor for Diabetic Nephropathy
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Clinical and Laboratory Measurements
4.2.1. Human Body Indices
4.2.2. Biochemical Indices
4.3. Assessment of Diabetic Nephropathy
4.4. Calculation of ABI
4.5. Diagnosis of NAFLD
4.6. Statistical Analysis
5. Conclusions
References
- Reid, A.E. Nonalcoholic steatohepatitis. Gastroenterology 2001, 121, 710–7232. [Google Scholar]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med 2002, 346, 1221–1231. [Google Scholar]
- McCullough, A.J. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol 2006, 40, S17–S29. [Google Scholar]
- Starley, B.Q.; Calcagno, C.J.; Harrison, S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010, 51, 1820–1832. [Google Scholar]
- Targher, G.; Bertolini, L.; Poli, F.; Rodella, S.; Scala, L.; Tessari, R.; Zenari, L.; Falezza, G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005, 54, 3541–3546. [Google Scholar]
- Targher, G.; Bertolini, L.; Rodella, S.; Tessari, R.; Zenari, L.; Lippi, G.; Arcaro, G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007, 30, 2119–2121. [Google Scholar]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Tessari, R.; Zenari, L.; Day, C.; Arcaro, G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007, 30, 1212–1218. [Google Scholar]
- Shibata, M.; Kihara, Y.; Taguchi, M.; Tashiro, M.; Otsuki, M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 2007, 30, 2940–2944. [Google Scholar]
- Adams, L.A.; Waters, O.R.; Knuiman, M.W.; Elliott, R.R.; Olynyk, J.K. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: An eleven-year follow-up study. Am. J. Gastroenterol 2009, 104, 861–867. [Google Scholar]
- Targher, G.; Chonchol, M.; Zoppini, G.; Abaterusso, C.; Bonora, E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: Is there a link? J. Hepatol 2011, 54, 1020–1029. [Google Scholar]
- Fugmann, T.; Borgia, B.; Révész, C.; Godó, M.; Forsblom, C.; Hamar, P.; Holthöfer, H.; Neri, D.; Roesli, C. Proteomic identification of vanin-1 as a marker of kidney damage in a rat model of type 1 diabetic nephropathy. Kidney Int 2011, 80, 272–281. [Google Scholar]
- Leitão, C.B.; Canani, L.H.; Bolson, P.B.; Molon, M.P.; Silveiro, S.P.; Gross, J.L. What values should be used to diagnose microalbuminuria in patients with diabetes mellitus? Arq. Bras. Endocrinol. Metabol 2006, 50, 322–326. [Google Scholar]
- Levin-Iaina, N.; Iaina, A.; Raz, I. The emerging role of NO and IGF-1 in early renal hypertrophy in STZ-induced diabetic rats. Diabetes Metab. Res. Rev 2011, 27, 235–243. [Google Scholar]
- Kumar, R.; Winocour, P.H. Dual blockade of the rennin-angiotensin system in diabetes—Rationale and risks. Br. J. Diabetes Vasc. Dis 2005, 5, 266–271. [Google Scholar]
- Medina, J.; Fernandez-Salazar, L.I.; Garcia-Buey, L.; Moreno-Otero, R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care 2004, 27, 2057–2066. [Google Scholar]
- Targher, G.; Bertolini, L.; Rodella, S.; Zoppini, G.; Lippi, G.; Day, C.; Muggeo, M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008, 51, 444–450. [Google Scholar]
- Hwang, S.T.; Cho, Y.K.; Yun, J.W.; Park, J.H.; Kim, H.J.; Park, D.I.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Rhee, E.J.; et al. Impact of NAFLD on microalbuminuria in patients with prediabetes and diabetes. Intern. Med. J 2010, 40, 437–442. [Google Scholar]
- Manco, M.; Ciampalini, P.; deVito, R.; Vania, A.; Cappa, M.; Nobili, V. Albuminuria and insulin resistance in children with biopsy proven non-alcoholic fatty liver disease. Pediatr. Nephrol 2009, 24, 1211–1217. [Google Scholar]
- Bloomgarden, Z.T. Diabetic Nephropathy. Diabetes Care 2005, 28, 745–751. [Google Scholar]
- Lakhotia, M.; Gehlot, R.S.; Jain, P.; Sharma, S.; Singh, M. Lipoprotein(a) in Type 2 Diabetic Subjects in relation to Diabetic Microvascular Complications. JIACM 2003, 4, 304–307. [Google Scholar]
- Farrell, G.C.; Chitturi, S.; Au, G.K.; Sollano, J.D. Asia-Pacific Working Party on NAFLD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: Executive summary. J. Gastroenterol. Hepatol 2007, 22, 775–777. [Google Scholar]
- Zeng, M.D.; Li, Y.M.; Chen, C.W.; Lu, L.G.; Fan, J.G.; Wang, B.Y.; Mao, Y.M. Guidelines for the diagnosis and treatment of alcoholic liver disease. J. Dig. Dis 2008, 9, 113–116. [Google Scholar]
| Variables | With NAFLD | Without NAFLD | t or χ2 | p |
|---|---|---|---|---|
| n | 202 | 161 | - | - |
| Sex (% men) | 40.0 | 49.10 | 2.92 (χ2) | 0.09 |
| Age (years) | 59.38 ± 11.43 | 60.55 ± 11.52 | 0.97 (t) | 0.33 |
| Duration of diabetes (years) | 8.95 ± 6.78 | 9.81 ± 7.29 | 1.17 (t) | 0.24 |
| BMI (kg/m2) | 26.76 ± 6.18 | 24.46 ± 14.88 | 8539.50 (Mann-Whitney U) | <0.001 |
| Waist circumference (cm) | 95.61 ± 10.20 | 87.44 ± 9.80 | −7.70 (t) | <0.001 |
| High blood pressure (%) | 39.6 | 49.1 | 2.66 (χ2) | 0.45 |
| UAER (μg/min) | 156.84 ± 526.30 | 231.18 ± 791.98 | 16071.00 (Mann-Whitney U) | 0.85 |
| FBG (mmol/L) | 8.03 ± 2.85 | 7.99 ± 2.89 | −0.13 (t) | 0.89 |
| HbA1c (%) | 8.72 ± 1.89 | 9.12 ± 2.38 | 1.59 (t) | 0.11 |
| TC (mmol/L) | 4.99 ± 1.08 | 4.97 ± 1.18 | −0.25 (t) | 0.8 |
| TG(mmol/L) | 2.06 ± 1.15 | 1.71 ± 1.30 | 11848.00 (Mann-Whitney U) | <0.001 |
| HDL (mmol/L) | 1.11 ± 0.30 | 1.30 ± 0.55 | 3.91 (t) | <0.001 |
| LDL (mmol/L) | 3.18 ± 0.93 | 3.12 ± 0.90 | −0.71 (t) | 0.48 |
| AST (U/L) | 24.59 ± 12.35 | 20.78 ± 6.61 | −3.76 (t) | <0.001 |
| ALT (U/L) | 22.95 ± 15.52 | 16.44 ± 6.91 | 11131.50 (Mann-Whitney U) | <0.001 |
| ABI (right) | 1.03 ± 0.16 | 1.01 ± 0.18 | 6017.50 (Mann-Whitney U) | 0.6 |
| ABI (left) | 1.06 ± 0.17 | 1.02 ± 0.20 | 5874.00 (Mann-Whitney U) | 0.41 |
| With NAFLD (n = 202) | Without NAFLD (n = 161) | χ2 | p | |
|---|---|---|---|---|
| Diabetic nephropathy | 75 (37.1%) | 62 (38.5%) | 0.073 | 0.787 |
| Microalbuminuria | 51 (25.2%) | 41 (25.5%) | 0.502 | 0.778 |
| Macroalbuminuria | 24 (11.9%) | 23 (14.3%) | 0.50 | 0.480 |
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Duration of diabetes | 1.065 | 1.014–1.120 | 0.012 |
| Waist circumference | 1.077 | 1.040–1.116 | <0.001 |
| FBG | 1.136 | 1.023–1.262 | 0.017 |
| SEX | 0.612 | 0.326–1.150 | NS |
| High blood pressure | 1.318 | 0.697–2.491 | NS |
| TG | 1.226 | 0.976–1.541 | NS |
| TC | 1.143 | 0.863–1.514 | NS |
| ABI (left) | 0.313 | 0.015–6.570 | NS |
| ABI (right) | 0.373 | 0.016–8.951 | NS |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhan, Y.-T.; Zhang, C.; Li, L.; Bi, C.-S.; Song, X.; Zhang, S.-T. Non-Alcoholic Fatty Liver Disease Is not Related to the Incidence of Diabetic Nephropathy in Type 2 Diabetes. Int. J. Mol. Sci. 2012, 13, 14698-14706. https://doi.org/10.3390/ijms131114698
Zhan Y-T, Zhang C, Li L, Bi C-S, Song X, Zhang S-T. Non-Alcoholic Fatty Liver Disease Is not Related to the Incidence of Diabetic Nephropathy in Type 2 Diabetes. International Journal of Molecular Sciences. 2012; 13(11):14698-14706. https://doi.org/10.3390/ijms131114698
Chicago/Turabian StyleZhan, Yu-Tao, Chuan Zhang, Li Li, Chun-Shan Bi, Xin Song, and Shu-Tian Zhang. 2012. "Non-Alcoholic Fatty Liver Disease Is not Related to the Incidence of Diabetic Nephropathy in Type 2 Diabetes" International Journal of Molecular Sciences 13, no. 11: 14698-14706. https://doi.org/10.3390/ijms131114698




