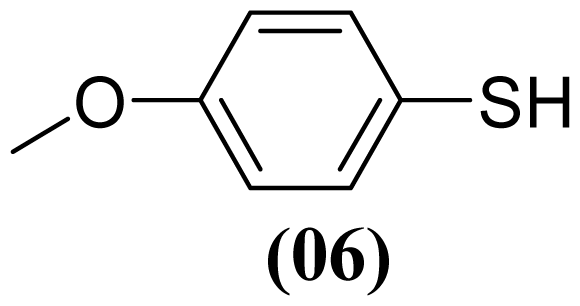
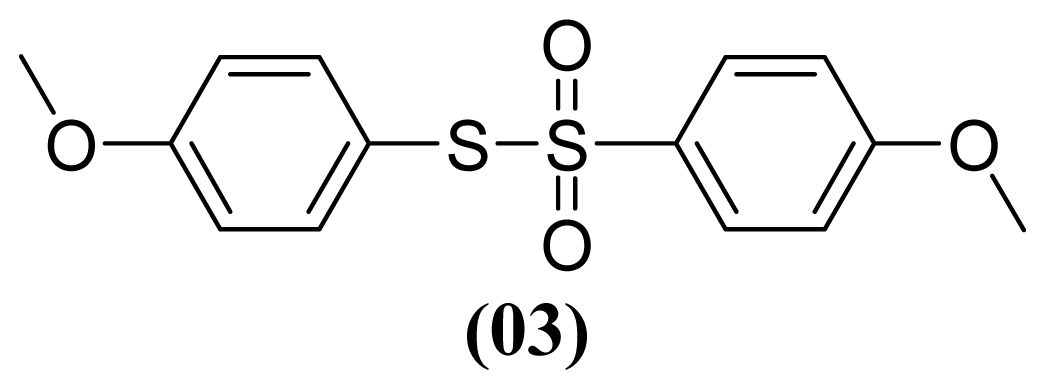
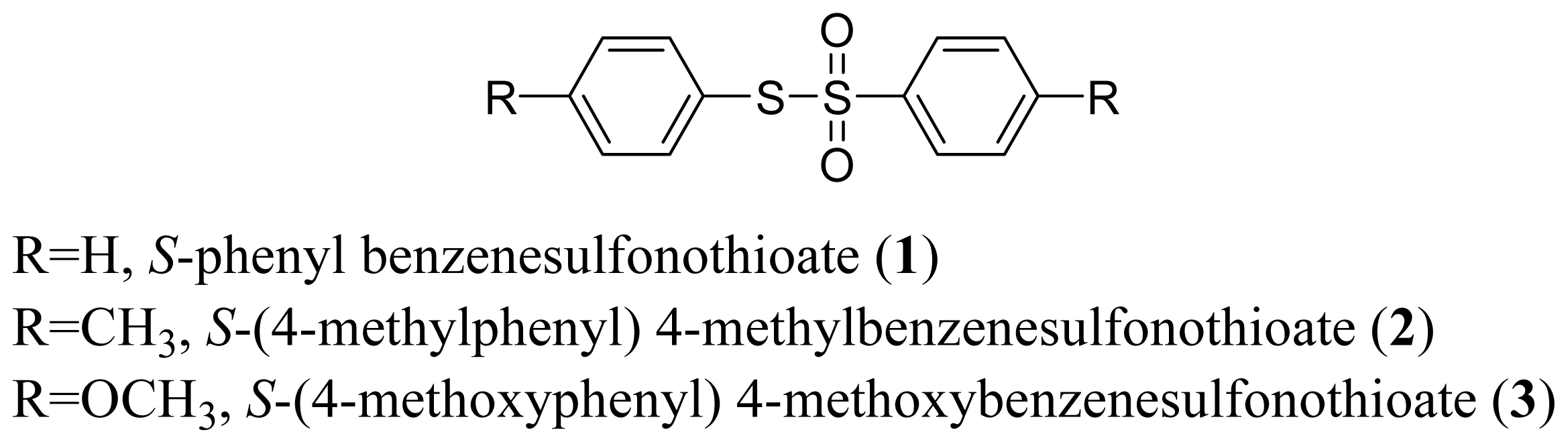
Synthesis Method for Thiosulfonate and Report of Its Insecticidal Activity in Anagasta kuehniella (Lepidoptera: Pyralidae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Methods of Chemistry
2.1.1. General Procedure with AgNO3/BF3·OEt2 and Al(H2PO4)3/HNO3
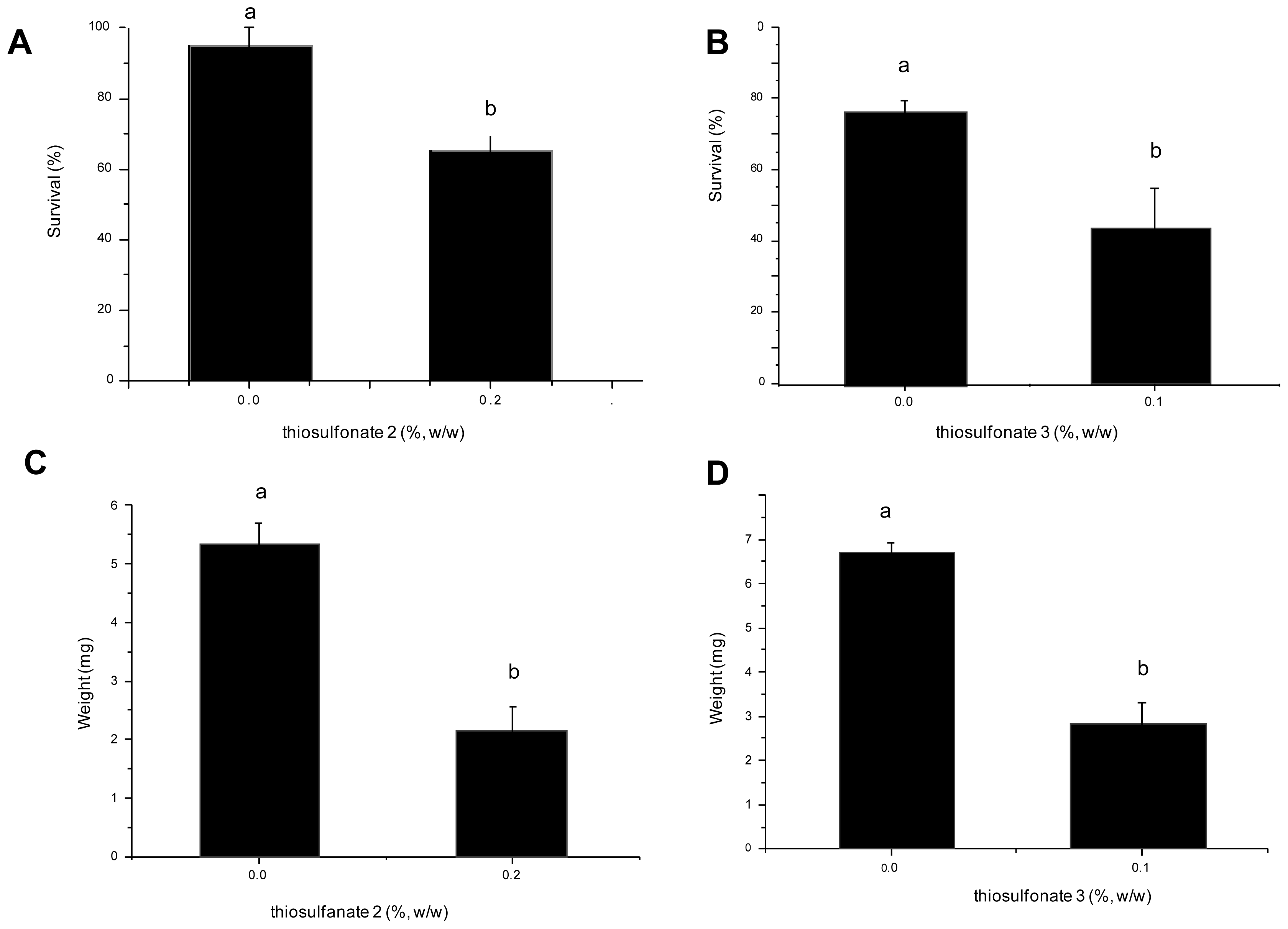
2.2. Effects of Thiosulfonates 2 and 3 on the Development of A. kuehniella
2.2.1. Nutritional Parameters
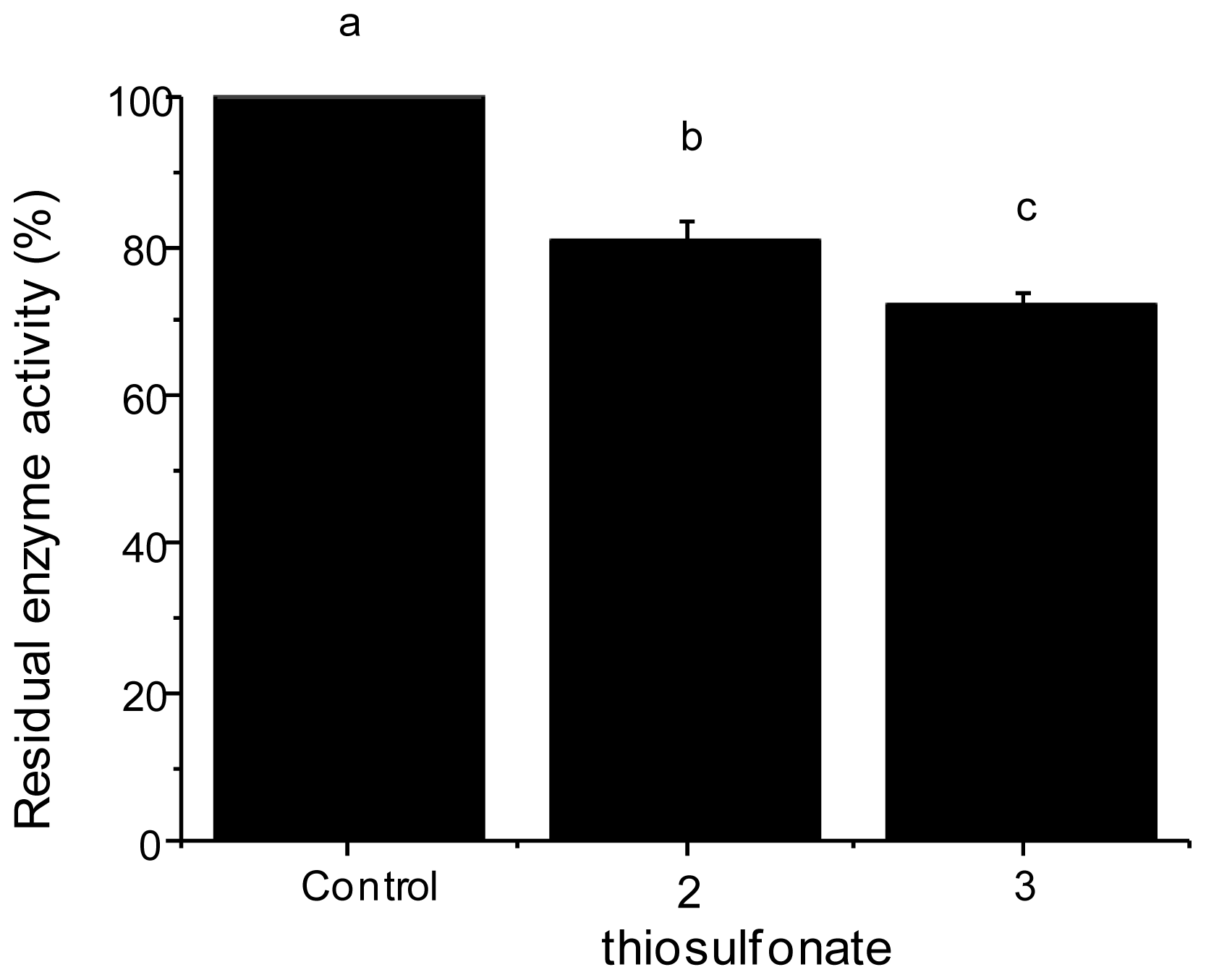
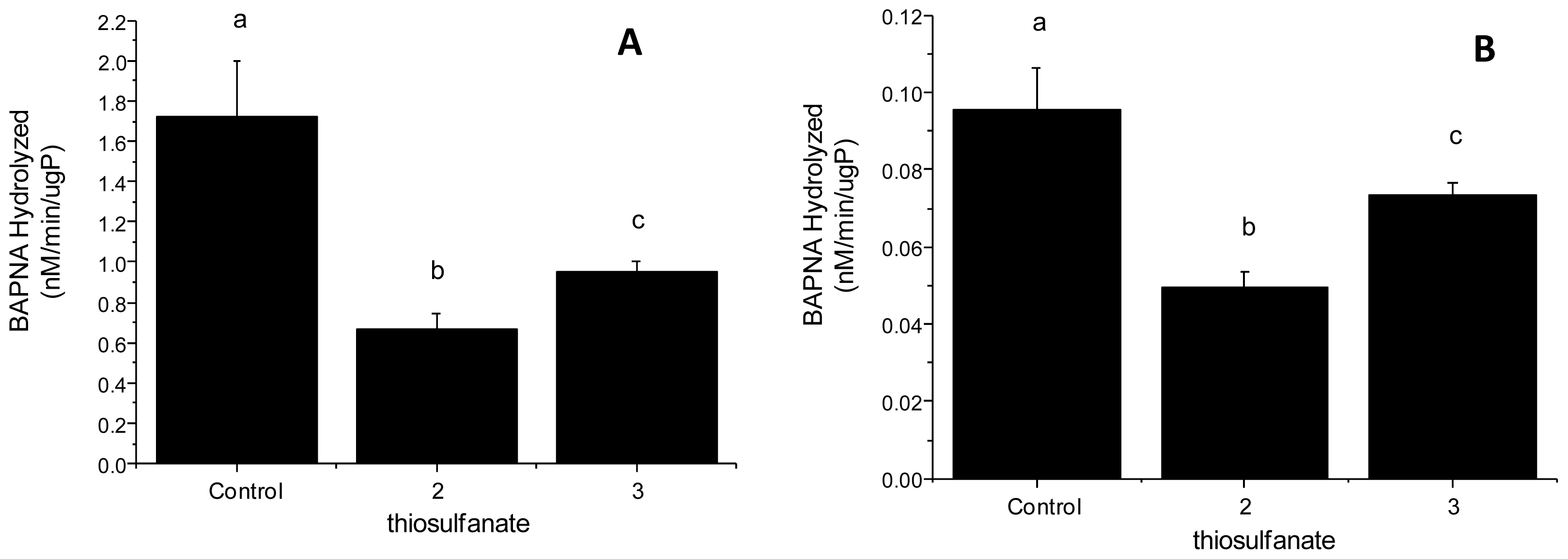
2.2.2. Tryptic Activity
3. Experimental Section
3.1. Experimental Methods of Chemistry
3.1.1. General Procedure with AgNO3/BF3·OEt2
3.1.2. General Procedure with Al(H2 PO4)3/HNO3
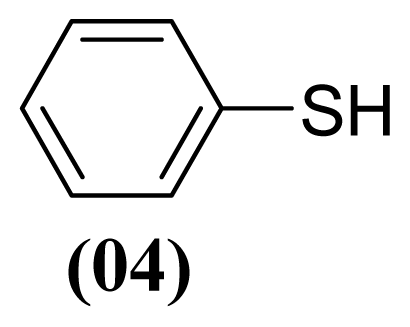
3.1.3. S-phenyl Benzenesulfonothioate (1)
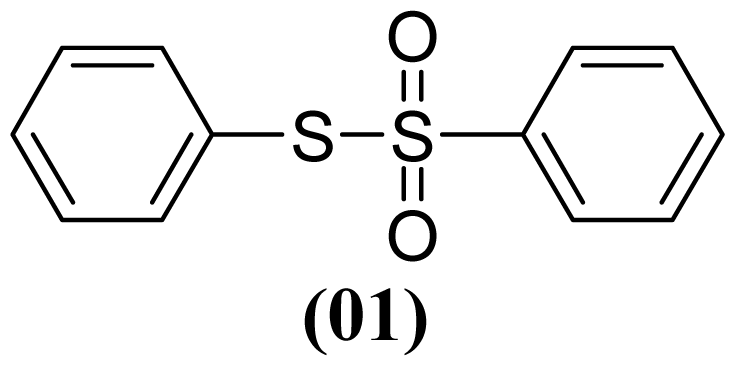
3.1.4. S-(4-Methylphenyl) 4-Methylbenzenesulfonothioate (2)
3.1.5. S-(4-Methoxyphenyl) 4-Methoxybenzenesulfonothioate (3)
3.2. Insect
3.2.1. Midgut Preparation
3.2.2. Fecal Pellet Preparations
3.2.3. Enzyme Assays
3.3. Effects of Thiosulfonates 2 and 3 on the Development of A. kuehniella
3.4. Nutritional Parameters
4. Conclusions
Acknowledgments
References
- Zhao, Y.Y.; Liu, F.; Yang, G.; You, M.S. PsOr1, a potential target for RNA interference-based pest management. Insect Mol. Biol 2011, 20, 97–104. [Google Scholar]
- Lorini, I.; Morás, A.; Beckel, H. Efeito inseticida de pós inertes no controle de pragas de grãos de trigo armazenado. Available online: http://www.cnpt.embrapa.br/biblio/p_co93.htm accessed on 17 April 2012.
- Macedo, M.L.R.; Durigan, R.A.; Silva, D.S.; Freire, M.G.M. Adenanthera pavonina trypsin inhibitor retard growth of Anagasta kuehniella (LEPIDOPTERA: PYRALIDAE). Arch. Insect Biochem. Physiol 2010, 73, 213–231. [Google Scholar]
- Coelho, M.B.; Marangoni, S.; Macedo, M.L.R. Insecticidal action of Annona coriacea lectin against the flour moth Anagasta kuehniella and the rice moth Corcyra cephalonica (Lepidoptera: Pyralidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol 2007, 146, 406–414. [Google Scholar]
- Macedo, M.L.R.; Freire, M.G.M.; Silva, M.B.R.; Coelho, L.C.B.B. Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculates (Coleoptera: Bruchidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol 2006, 146, 486–498. [Google Scholar]
- Fields, P.G. Effect of Pisum sativum fractions on the mortality and progeny production of nine stored-grain beetles. J. Stored. Products Res 2006, 42, 86–96. [Google Scholar]
- Wang, H.; Mao, Y.; Chen, A.Y.; Zhou, N.; Lavoie, E.J.; Liu, L.F. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry 2001, 40, 3316–3323. [Google Scholar]
- Baerlocher, F.J.; Baerlocher, M.O.; Chaulk, C.L.; Langler, R.F.; MacQuarrie, S.L. Antifungal thiosulfonates: Potency with some selectivity. Aust. J. Chem 2000, 53, 399–402. [Google Scholar]
- Kretschik, O.; Kugler, M.; Jaetsch, T. Thiosulfonic acid S-esters as agent for protecting material US 20020151570, 17 October 2002.
- Konishi, K. New insecticidally active derivatives of nereistoxin. Agric. Biol. Chem 1968, 32, 678–679. [Google Scholar]
- Konishi, K. Studies on organic insecticides XIII synthesis of nereistoxin and related compounds VI. Agric. Biol. Chem 1970, 34, 1549–1560. [Google Scholar]
- Bandgar, B.P.; Pandit, S.S. Direct synthesis of thiosulfonic S-esters from sulfonic acids using cyanuric chloride under mild conditions. J. Sulfur. Chem 2004, 25, 347–350. [Google Scholar]
- Oae, S.; Togo, H.; Numata, T.; Fujimori, K. Facile reduction of sulfinic acid to disulfide with thiol and chlorotrimethylsilane. Chem. Lett 1980, 9, 1193–1196. [Google Scholar]
- Mahieu, J.P.; Gosselet, M.; Sebille, B.; Beuzard, Y. Synthesis of new thiosulfonates and disulfides from sulfonyl chlorides and thiols. Synth. Commun 1986, 16, 1709–1722. [Google Scholar]
- Bonifácio, V.D.B.; Morgado, J.; Scherf, U. Synthesis of thiosulfonate-briged bromofluorene end-capping reagents. Synlett 2010, 9, 1333–1336. [Google Scholar]
- Cai, M.T.; Lv, G.S.; Chen, J.X.; Gao, W.X.; Ding, J.C.; Wu, H.Y. CAN/I2-catalyzed chemoselective synthesis of thiosulfonates by oxidation of disulfides or thiols. Chem. Lett 2010, 39, 368–369. [Google Scholar]
- Iranpoor, N.; Mohajer, D.; Rezaeifard, A.R. Rapid and highly chemoselective biomimetic oxidation of organosulfur compounds with tetrabutylammonium peroxymonosulfate in the presence of manganese meso-tetraphenylporphyrin and imidazole. Tetrahedron Lett 2004, 45, 3811–3815. [Google Scholar]
- Novo Lesnugin, A.Z.; Anisimov, A.V.; Tarakanova, A.V. Oxidation of thiols with hydrogen peroxide under phase transfer catalysis conditions. Neftekhimiya 2000, 40, 462–464. [Google Scholar]
- Bharadwaj, S.K.; Hussain, S.; Kar, M.; Chaudhuri, M.K. Al(H2PO4)3: An efficient catalyst for nitration of organic compounds with nitric acid. Catal. Commun 2008, 9, 919–923. [Google Scholar]
- Olah, G.A.; Fung, A.P.; Narang, S.C.; Olah, J.A. Aromatic substitution. 48. boron trifluoride catalyzed nitration of aromatics with silver nitrate in acetonitrile solution. J. Org. Chem 1981, 46, 3533–3537. [Google Scholar]
- Goswami, P.; Bharadwaj, S.K. Al(H2PO4)3: An efficient and effective solid acid catalyst for transesterification of β-keto esters under solvent free condition. Catal. Lett 2008, 124, 100–104. [Google Scholar]
- Nathan, S.S.; Kalaivani, K. Efficacy of nucleopolyhedrovirus (NPV) and azadirachtin on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Biol. Control 2005, 34, 93–98. [Google Scholar]
- Nathan, S.S.; Kalaivani, K.; Murugan, K.; Chung, P.G. Efficacy of neem limonoids on Cnaphalocrocis medicinalis (Guenée) (Lepidoptera: Pyralidae) the rice leaf holder. Crop. Prot 2005, 24, 760–763. [Google Scholar]
- Ramos, V.S.; Silva, G.S.; Freire, M.G.M.; Parra, J.R.P.; Macedo, M.L.R. Purification and characterization of a trypsin inhibitor from Plathymenia foliolosa seeds. J. Agric. Food. Chem 2008, 10, 11348–11355. [Google Scholar]
- Wheeler, D.A.; Isman, M.B. Antifeedant and toxic activity of Trichilia americana extract against the larvae of Spodoptera litura. Entomol. Exp. Appl 2001, 98, 9–16. [Google Scholar]
- Macedo, M.L.R.; De Sá, C.M.; Freire, M.G.M.; Parra, J.R.P. A Kunitz-type inhibitor of coleopteran proteases, isolated from Adenanthera pavonina L. seeds and its effect on Callosobruchus maculates. J. Agric. Food Chem 2004, 52, 2533–2540. [Google Scholar]
- Tsilikounas, E.; Rao, T.; Gutheil, W.G.; Bachovchin, W.W. 15N and 1H NMR spectroscopy of the catalytic histidine in chloromethyl ketone-inhibited complexes of serine proteases. Biochemistry 1996, 35, 2437–2444. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed; Oxford: New York, NY, USA; p. 1988.
- Quesada, M.L.; Schlessinger, R.H. A convenient preparation of methyl 2,5-dihydro-2-oxo-3- furancarboxylate. J. Org. Chem 1978, 43, 346–347. [Google Scholar]
- Carson, J.F.; Wong, F.F. The reactions of thiolsulfonates and thiolsulfinates with l-FIuoro-2,4- dinitrobenzene. J. Org. Chem 1961, 26, 3028–3030. [Google Scholar]
- Xu, Y.; Peng, Y.; Sun, J.; Chen, J.; Ding, J.; Wu, H. CCA-promoted solvent-free chemoselective synthesis of thiosulfonates on grinding. J. Chem. Res 2010, 34, 358–360. [Google Scholar]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two chromogenic substrates of trypsin. Arch. Biochem. Biophys 1961, 95, 271–278. [Google Scholar]
- Farrar, R.R.; Barbour, J.D.; Kenedy, G.G. Quantifying food consumption and growth in insects. Ann. Entomol. Soc. Am 1989, 82, 593–598. [Google Scholar]
- Waldbauer, G.P. The consumption and utilization of food by insects. Adv. Insect Physiol 1968, 5, 229–288. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dos Santos, E.D.A.; Gonçalves, F.M.; Prado, P.C.; Sasaki, D.Y.; De Lima, D.P.; Macedo, M.L.R. Synthesis Method for Thiosulfonate and Report of Its Insecticidal Activity in Anagasta kuehniella (Lepidoptera: Pyralidae). Int. J. Mol. Sci. 2012, 13, 15241-15251. https://doi.org/10.3390/ijms131115241
Dos Santos EDA, Gonçalves FM, Prado PC, Sasaki DY, De Lima DP, Macedo MLR. Synthesis Method for Thiosulfonate and Report of Its Insecticidal Activity in Anagasta kuehniella (Lepidoptera: Pyralidae). International Journal of Molecular Sciences. 2012; 13(11):15241-15251. https://doi.org/10.3390/ijms131115241
Chicago/Turabian StyleDos Santos, Edson Dos A., Fernando M. Gonçalves, Paulo César Prado, Daniele Y. Sasaki, Dênis P. De Lima, and Maria Lígia Rodrigues Macedo. 2012. "Synthesis Method for Thiosulfonate and Report of Its Insecticidal Activity in Anagasta kuehniella (Lepidoptera: Pyralidae)" International Journal of Molecular Sciences 13, no. 11: 15241-15251. https://doi.org/10.3390/ijms131115241