Simple Estimation of Förster Resonance Energy Transfer (FRET) Orientation Factor Distribution in Membranes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Special Theoretical Cases
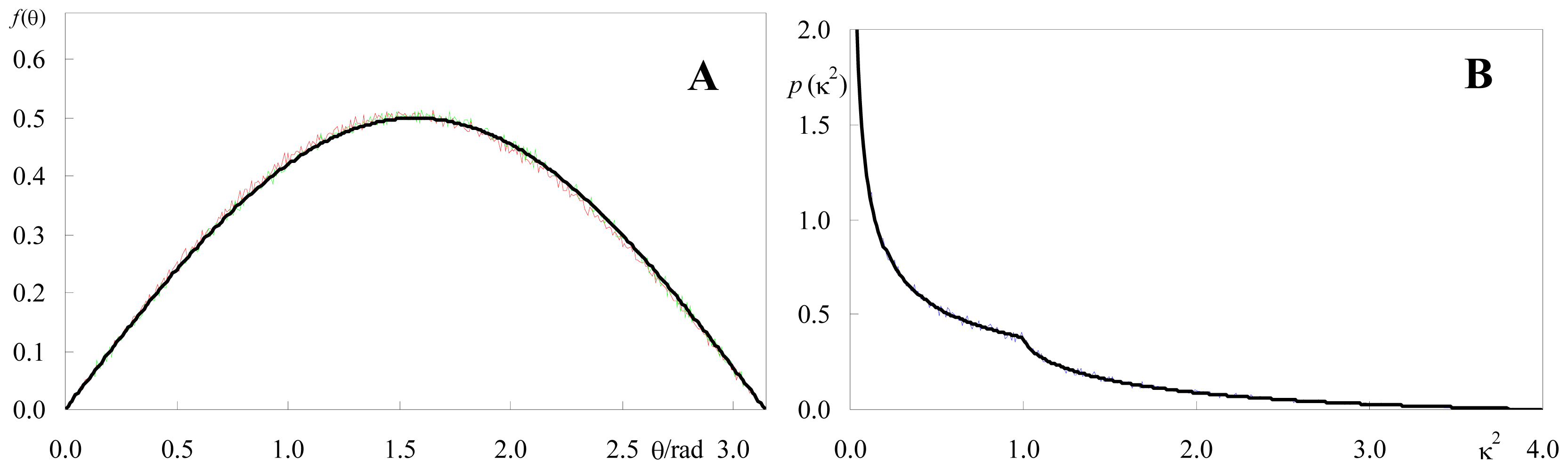
2.1.1. Isotropic Dipole Distribution
2.1.2. Identical or Identically-Distributed Fluorophores
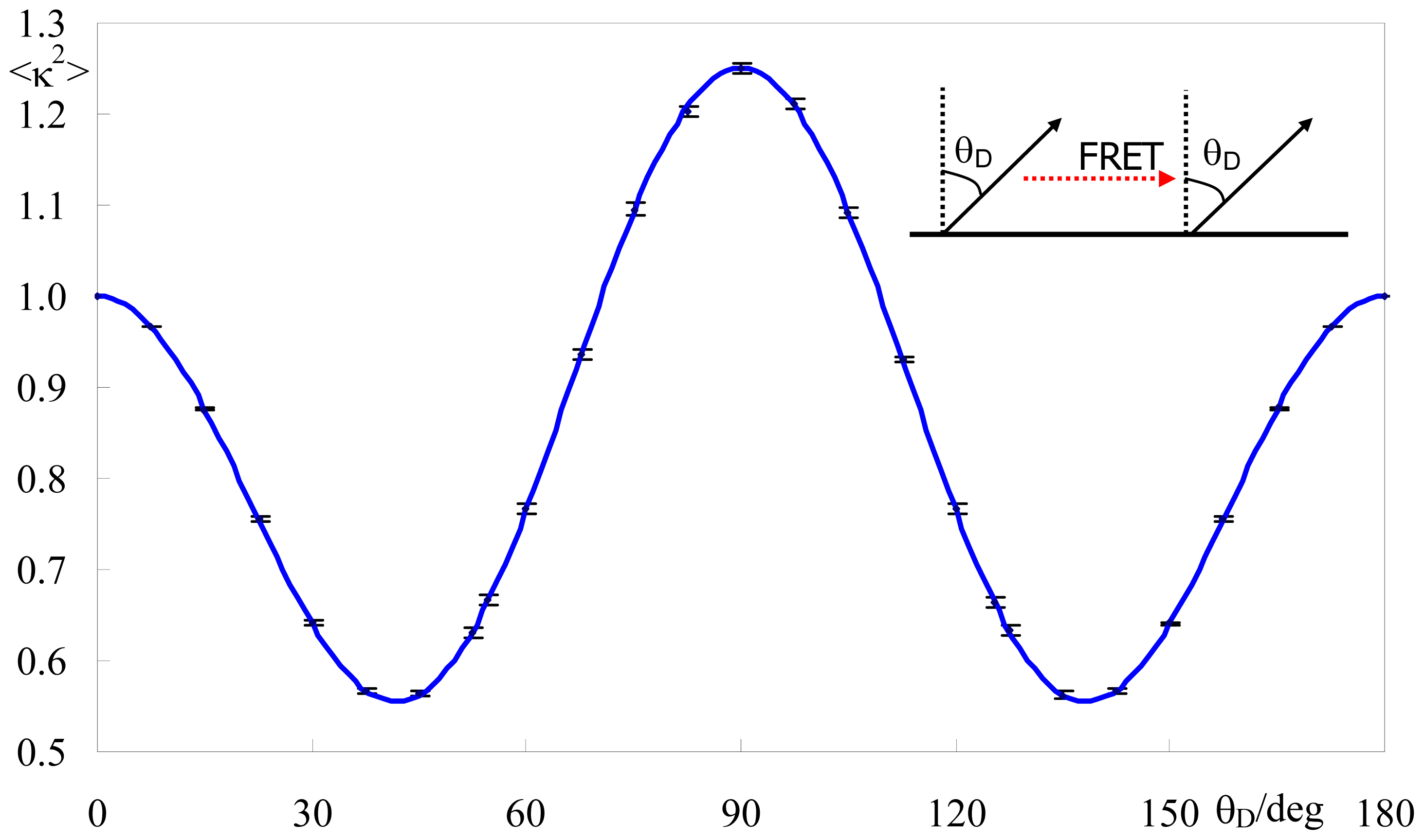
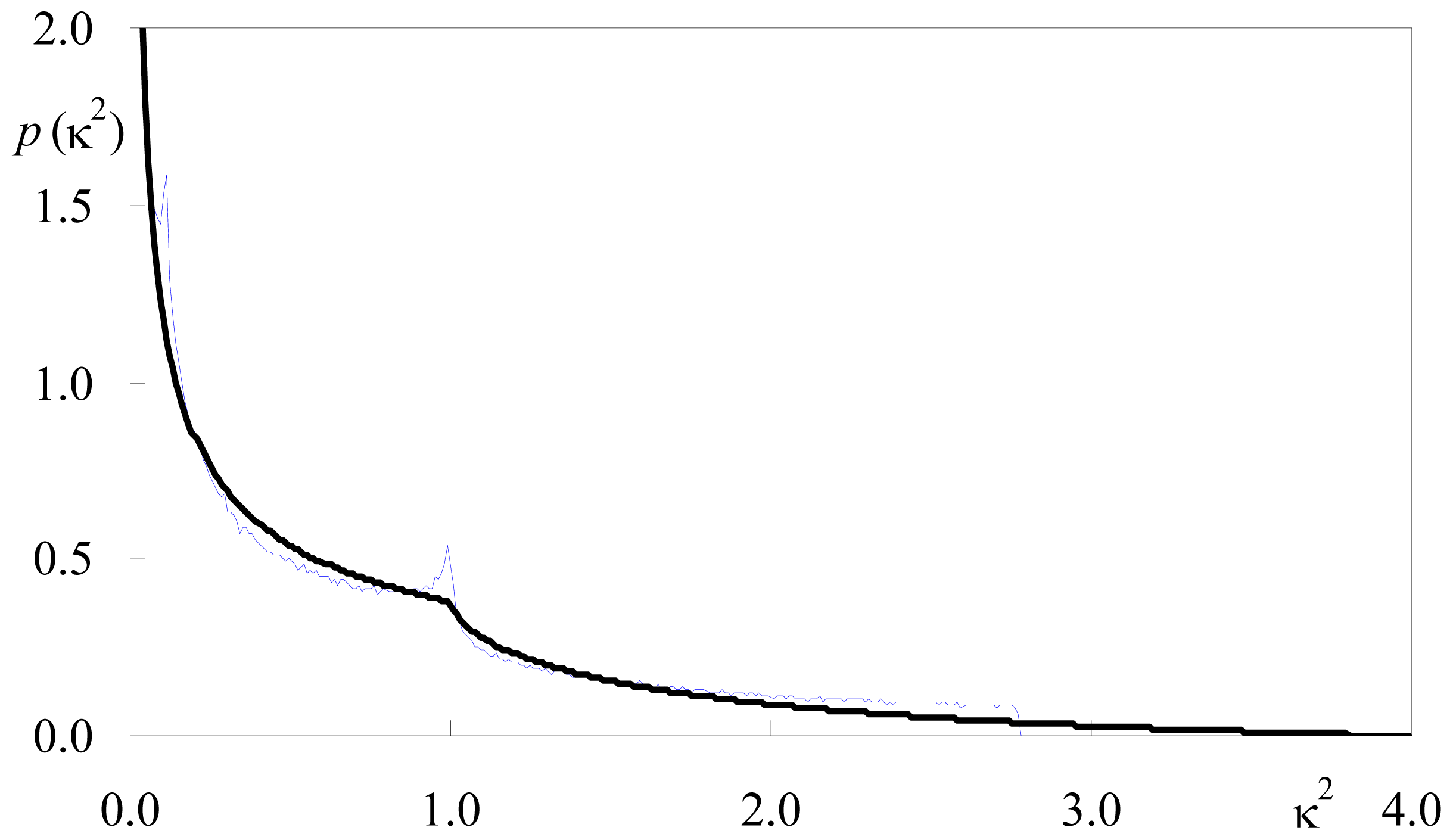
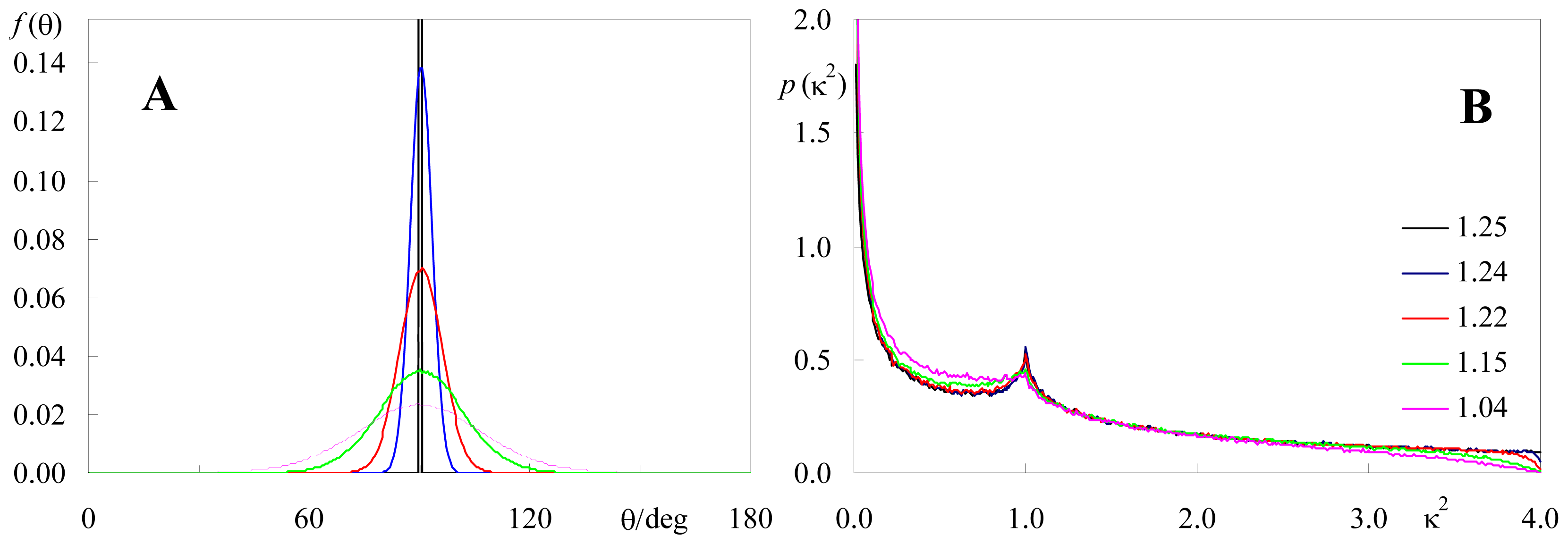
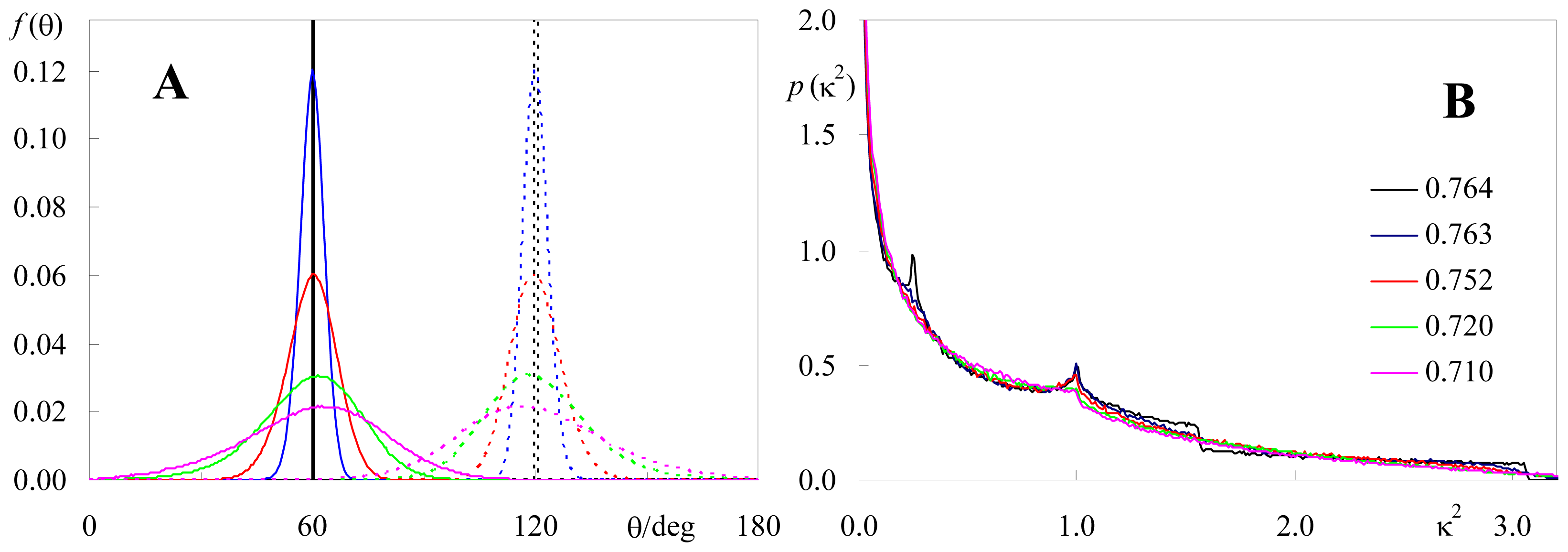
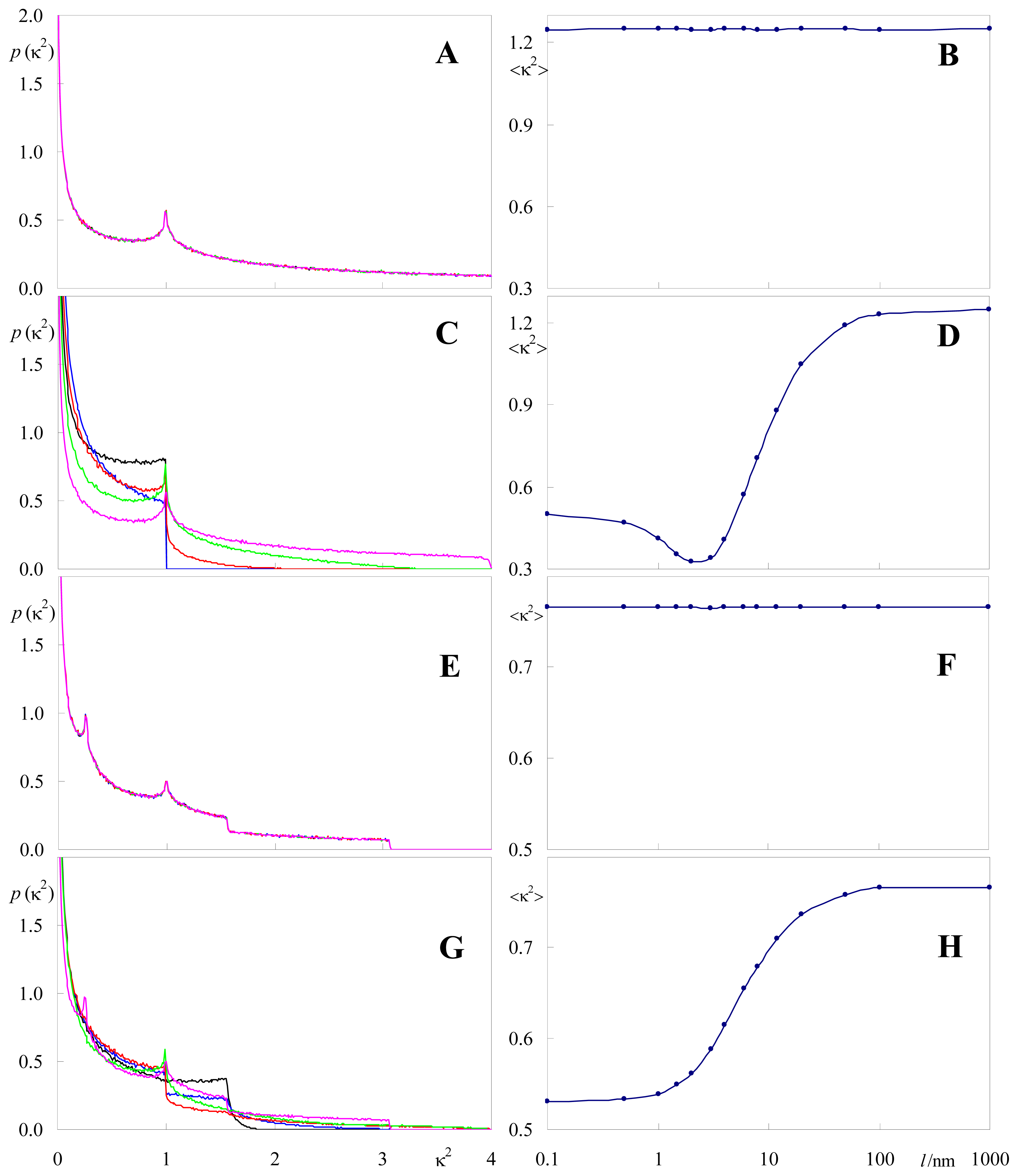
2.2. Effect of Orientation Heterogeneity
2.3. Distance Effects
2.4. Application to Experimental FRET Pairs
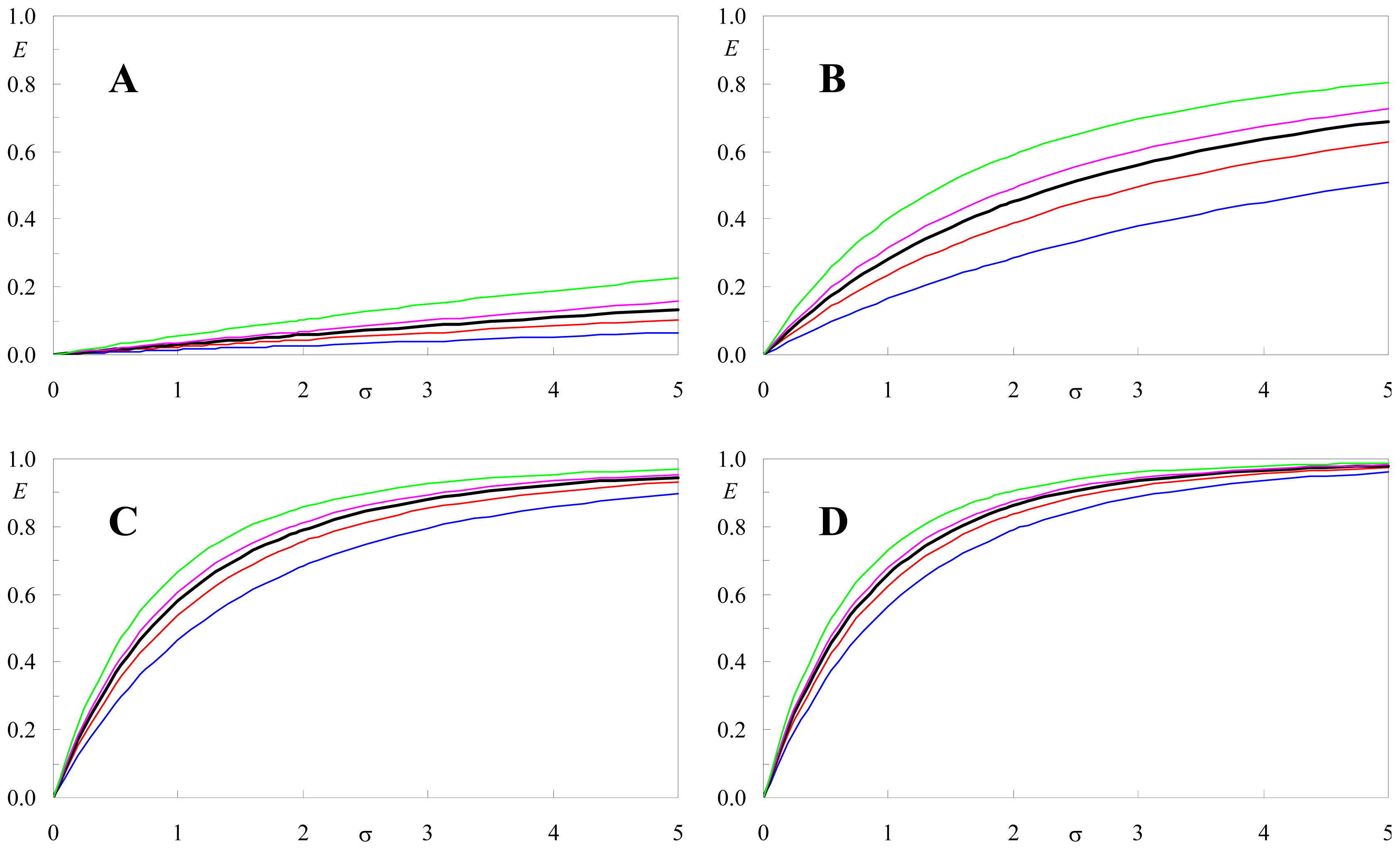
2.5. Effect of <κ2> on FRET Efficiency
3. Methods
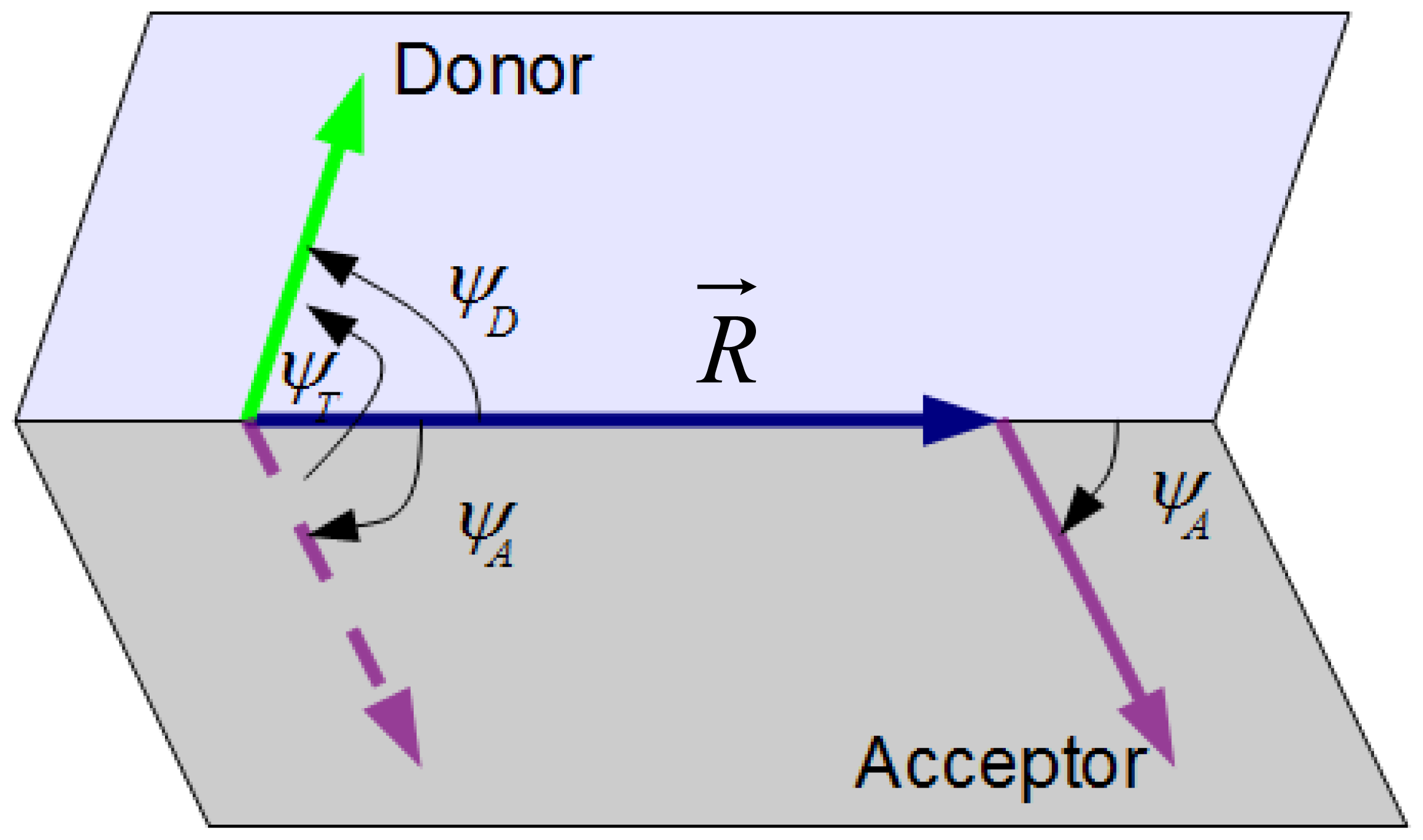
3.1. Definition of κ2 for a Given Donor-Acceptor Pair
3.2. Calculation of κ2 Distributions and Averages from Numerical Simulation
- Read inputs: θD,max, θA,max, σD, σA, l, N, donor-acceptor interplanar distance h, and the number of bins (equal length subintervals of 0 ≤ κ2 ≤ 4) for κ2 distribution.
- Main cycle – calculate κ2 for the ith donor-acceptor pair:
- 2.1. Set donor and acceptor coordinates, (xDi, yDi, zDi) and (xAi, yAi,zAi), respectively. For the x and y-coordinates, uniformly distributed random numbers between 0 and L are allotted. zDi is taken as zero, whereas zAi is taken as h.
- 2.2. Calculate donor-acceptor separating vector R⃗, and its modulus.
- 2.3. Set donor and acceptor dipole orientation coordinates, (θDi, ϕDi) and (θAi, ϕAi), respectively. For ϕDi and ϕAi, uniformly distributed random numbers between 0 and 2π are allotted. θDi and θAi (the angles relative to the bilayer normal, so that they fall between 0 and π) are calculated by firstly computing their cosines, using Gaussian random numbers centered around cosθD,max and cosθA,max, with standard deviation σD and σA, respectively. Gaussian random numbers are obtained using the Box-Muller transform method [51,52]. If either of the calculated cosines falls outside the [-1,1] interval, new cosθDi and cosθAi are calculated. Otherwise, θDi and θAi are obtained in the [0, π] interval by inversion.
- 2.4. Calculate Cartesian coordinates of unit dipole vectors u⃗D and u⃗A, from (θDi, ϕDi) and (θAi, ϕAi).
- 2.5. Calculate κ2 using Equation 12.
- 2.6. Update histogram of κ2 distribution (in fact, the program also calculates θD and θA distributions, for testing and/or comparison and matching with distributions from the literature; these are also updated at this point), and partial sum of κ2 values (for calculation of <κ2> = sum(κ2i)/N at the end of the cycle).
- 2.7 Increment i and repeat until i > N.
- Write outputs to the active worksheet: κ2 average value and distribution, θD and θA distributions.
4. Conclusions
Acknowledgments
References
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed; Springer: New York, NY, USA, 2006. [Google Scholar]
- Loura, L.M.S.; Prieto, M. FRET in membrane biophysics: An overview. Front Physiol 2011, 2, 82. [Google Scholar]
- De Almeida, R.F.; Loura, L.M.S.; Prieto, M. Membrane lipid domains and rafts: Current applications of fluorescence lifetime spectroscopy and imaging. Chem. Phys. Lipids 2009, 157, 61–77. [Google Scholar]
- Dale, R.E.; Eisinger, J.; Blumberg, W.E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys. J 1979, 26, 161–193. [Google Scholar]
- Van der Meer, B.W. Kappa-squared: From nuisance to new sense. Rev. Mol. Biotechnol 2002, 82, 181–196. [Google Scholar]
- VanBeek, D.B.; Zwier, M.C.; Shorb, J.M.; Krueger, B.P. Fretting about FRET: Correlation between κ and R. Biophys. J 2007, 92, 4168–4178. [Google Scholar]
- Allen, L.R.; Paci, E. Orientational averaging of dye molecules attached to proteins in Förster resonance energy transfer measurements: Insights from a simulation study. J. Chem. Phys 2009, 131, 065101. [Google Scholar]
- Hoefling, M.; Lima, N.; Haenni, D.; Seidel, C.A.M.; Schuler, B.; Grubmüller, H. Structural heterogeneity and quantitative FRET efficiency distributions of polyprolines through a hybrid atomistic simulation and Monte Carlo approach. PLoS One 2011, 6, e19791. [Google Scholar]
- Deplazes, E.; Jayatilaka, D.; Corry, B. Testing the use of molecular dynamics to simulate fluorophore motions and FRET. Phys. Chem. Chem. Phys 2011, 13, 11045–11054. [Google Scholar]
- Loura, L.M.S.; Palace Carvalho, A.J.; Prates Ramalho, J.P. Direct calculation of Förster orientation factor of membrane probes by molecular simulation. J. Mol. Struct. THEOCHEM 2010, 946, 107–112. [Google Scholar]
- Loura, L.M.S.; Prates Ramalho, J.P. Fluorescent membrane probes’ behaviour in lipid bilayers: Insights from molecular dynamics simulations. Biophys. Rev 2009, 1, 141–148. [Google Scholar]
- Loura, L.M.S.; Prates Ramalho, J.P. Recent developments in molecular dynamics simulations of fluorescent membrane probes. Molecules 2011, 16, 5437–5452. [Google Scholar]
- Hicks, M.R.; Kowałski, J.; Rodger, A. LD spectroscopy of natural and synthetic biomaterials. Chem. Soc. Rev 2010, 39, 3380–3393. [Google Scholar]
- Lopes, S.C.D.N.; Castanho, M.A.R.B. Overview of common spectroscopic methods to determine the orientation/alignment of membrane probes and drugs in lipidic bilayers. Curr. Org. Chem 2005, 9, 889–898. [Google Scholar]
- Oreopoulos, J.; Yip, C.M. Probing Membrane order and topography in supported lipid bilayers by combined polarized total internal reflection fluorescence-atomic force microscopy. Biophys. J 2009, 96, 1970–1984. [Google Scholar]
- Beausang, J.F.; Sun, Y.; Quinlan, M.E.; Forkey, J.N.; Goldman, Y.E. Orientation and Rotational Motions of Single Molecules by Polarized Total Internal Reflection Fluorescence Microscopy (polTIRFM). Cold Spring Harb. Protoc. 2012. [Google Scholar] [CrossRef]
- Corry, B.; Jayatilaka, D.; Martinac, B.; Rigby, P. Determination of the orientational distribution and orientation factor for transfer between membrane-bound fluorophores using a confocal microscope. Biophys. J. 2006, 91, 1032–1045. [Google Scholar]
- Baumann, J.; Fayer, M.D. Excitation transfer in disordered two-dimensional and anisotropic three-dimensional systems: Effects of spatial geometry on time-resolved observables. J. Chem. Phys 1986, 85, 4087–4107. [Google Scholar]
- Knoester, J.; van Himbergen, J.E. Effect of molecular reorientation on excitation decay due to incoherent energy transfer. J. Chem. Phys 1984, 81, 4380–4388. [Google Scholar]
- Loura, L.M.S.; Prates Ramalho, J.P. Location and dynamics of acyl chain NBD-labeled phosphatidylcholine (NBD-PC) in DPPC bilayers. A molecular dynamics and time-resolved fluorescence anisotropy study. Biochim. Biophys. Acta 2007, 1768, 467–478. [Google Scholar]
- Kinosita, K.; Kawato, S.; Ikegami, A. A theory of fluorescence polarization decay in membranes. Biophys. J 1977, 20, 289–305. [Google Scholar]
- Fernandes, M.X.; García de la Torre, J.; Castanho, M.A.R.B. A brownian dynamics simulation of an acyl chain and a trans-parinaric acid molecule confined in a phospholipid bilayer in the gel and liquid-crystal phases. J. Phys. Chem. B 2000, 104, 11579–11584. [Google Scholar]
- Wolber, P.K.; Hudson, B.S. An analytic solution to the Förster energy transfer problem in two dimensions. Biophys. J 1979, 28, 197–210. [Google Scholar]
- Silva, L.; de Almeida, R.F.M.; Fedorov, A.; Matos, A.P.; Prieto, M. Ceramide-platform formation and -induced biophysical changes in a fluid phospholipid membrane. Mol. Membr. Biol 2006, 23, 137–148. [Google Scholar]
- Silva, L.C.; de Almeida, R.F.M.; Castro, B.M.; Fedorov, A.; Prieto, M. Formation of ceramide/sphingomyelin gel domains in the presence of an unsaturated phospholipid: A quantitative multiprobe approach. Biophys. J 2007, 93, 1639–1650. [Google Scholar]
- Fernandes, M.X.; García de la Torre, J.; Castanho, M.A.R.B. Joint determination by Brownian dynamics and fluorescence quenching of the in-depth location profile of biomolecules in membranes. Anal. Biochem 2002, 307, 1–12. [Google Scholar]
- Repáková, J.; Čapková, P.; Holopainen, J.M.; Vattulainen, I. Distribution, orientation, and dynamics of DPH probes in DPPC bilayer. J. Phys. Chem. B 2004, 108, 13438–13448. [Google Scholar]
- Loura, L.M.S.; Coutinho, A.; Silva, A.; Fedorov, A.; Prieto, M. Structural effects of a basic peptide on the organization of dipalmitoylphosphatidylcholine/dipalmitoylphosphatidylserine membranes: A fluorescent resonance energy transfer study. J. Phys. Chem. B 2006, 110, 8130–8141. [Google Scholar]
- Fernandes, F.; Loura, L.M.S.; Fedorov, A.; Prieto, M. Absence of clustering of phosphatidylinositol-(4,5)-bisphosphate in fluid phosphatidylcholine. J. Lipid Res 2006, 47, 1521–1525. [Google Scholar]
- Coutinho, A.; Loura, L.M.S.; Prieto, M. FRET studies of lipid-protein aggregates related to amyloid-like fibers. J. Neurochem 2011, 116, 696–701. [Google Scholar]
- Loura, L.M.S. Lateral distribution of NBD-PC fluorescent lipid analogs in membranes probed by molecular dynamics-assisted analysis of Förster Resonance Energy Transfer (FRET) and fluorescence quenching. Int. J. Mol. Sci 2012, 13, 14545–14564. [Google Scholar]
- Chattopadhyay, A.; London, E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry 1987, 26, 39–45. [Google Scholar]
- Huster, D.; Müller, P.; Arnold, K.; Herrmann, A. Dynamics of membrane penetration of the fluorescent 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) group attached to an acyl chain of phosphatidylcholine. Biophys. J 2001, 80, 822–831. [Google Scholar]
- Prieto, M.J.E.; Castanho, M.; Coutinho, A.; Ortiz, A.; Aranda, F.J.; Gómez-Fernández, J.C. Fluorescence study of a derivatized diacylglycerol incorporated in model membranes. Chem. Phys. Lipids 1994, 69, 75–85. [Google Scholar]
- Loura, L.M.S.; Fedorov, A.; Prieto, M. Membrane probe distribution heterogeneity: A resonance energy transfer study. J. Phys. Chem. B 2000, 104, 6920–6931. [Google Scholar]
- Loura, L.M.S.; Fedorov, A.; Prieto, M. Partition of membrane probes in a gel/fluid two-component lipid system: A fluorescence resonance energy transfer study. Biochim. Biophys. Acta 2000, 1467, 101–112. [Google Scholar]
- Gullapalli, R.R.; Demirel, M.C.; Butler, P.J. Molecular dynamics simulations of DiI-C18(3) in a DPPC lipid bilayer. Phys. Chem. Chem. Phys 2008, 10, 3548–3560. [Google Scholar]
- Lantzsch, G.; Binder, H.; Heerklotz, H. Surface area per molecule in lipid/C12En membranes as seen by fluorescence resonance energy transfer. J. Fluoresc 1994, 4, 339–343. [Google Scholar]
- Loura, L.M.S.; Fedorov, A.; Prieto, M. Exclusion of a cholesterol analog from the cholesterol-rich phase in model membranes. Biochim. Biophys. Acta 2001, 1511, 236–243. [Google Scholar]
- Loura, L.M.S.; Fedorov, A.; Prieto, M. Fluid-fluid membrane microheterogeneity: A fluorescence resonance energy transfer study. Biophys. J 2001, 80, 776–788. [Google Scholar]
- De Almeida, R.F.M.; Loura, L.M.S.; Fedorov, A.; Prieto, M. Lipid rafts have different sizes depending on membrane composition: A time-resolved fluorescence resonance energy transfer study. J. Mol. Biol 2005, 346, 1109–1120. [Google Scholar]
- Castro, B.M.; de Almeida, R.F.M.; Goormaghtigh, E.; Fedorov, A.; Prieto, M. Organization and dynamics of Fas transmembrane domain in raft membranes and modulation by ceramide. Biophys. J 2011, 101, 1632–1641. [Google Scholar]
- Medhage, B.; Mukhtar, E.; Kalman, B.; Johansson, L.B.-A.; Molotkovsky, J.G. Electronic energy transfer in anisotropic systems. Part 5—Rhodamine-lipid derivatives in model membranes. J. Chem. Soc. Faraday Trans 1992, 88, 2845–2851. [Google Scholar]
- Kyrychenko, A. A molecular dynamics model of rhodamine-labeled phospholipid incorporated into a lipid bilayer. Chem. Phys. Lett 2010, 485, 95–99. [Google Scholar]
- Johansson, L.B.-A.; Niemi, A. Electronic energy transfer in anisotropic systems. 1. Octadecylrhodamine B in vesicles. J. Phys. Chem 1987, 91, 3020–3023. [Google Scholar]
- Coutinho, A.; Loura, L.M.S.; Fedorov, A.; Prieto, M. Pinched multilamellar structure of aggregates of lysozyme and phosphatidylserine-containing membranes revealed by FRET. Biophys. J 2008, 95, 4726–4736. [Google Scholar]
- Song, K.C.; Livanec, P.W.; Klauda, J.B.; Kuczera, K.; Dunn, R.C.; Im, W. Orientation of fluorescent lipid analogue BODIPY-PC to probe lipid membrane properties: Insights from molecular dynamics simulations. J. Phys. Chem. B 2011, 115, 6157–6165. [Google Scholar]
- Heberle, F.A.; Wu, J.; Goh, S.L.; Petruzielo, R.S.; Feigenson, G.W. Comparison of three ternary lipid bilayer mixtures: FRET and ESR reveal nanodomains. Biophys. J 2010, 99, 3309–3318. [Google Scholar]
- Loura, L.M.S.; Fedorov, A.; Prieto, M. Resonance energy transfer in a model system of membranes: Application to gel and liquid crystalline phases. Biophys. J. 1996, 71, 1823–1836. [Google Scholar]
- Castro, B.M.; de Almeida, R.F.M.; Fedorov, A.; Prieto, M. The photophysics of a Rhodamine head labeled phospholipid in the identification and characterization of membrane lipid phases. Chem. Phys. Lipids 2012, 165, 311–319. [Google Scholar]
- Box, G.E.P.; Muller, M.E. A note on the generation of random normal deviates. Ann. Math. Stat 1958, 29, 610–611. [Google Scholar]
- Pike, M.C. Algorithm 267: Random normal deviate [G5]. Comm. ACM 1965, 8, 606. [Google Scholar]
| Donor fluorophore a | Acceptor fluorophore a | Quantitative experimental FRET studies | Donor transverse location b | Donor orientation (θmax, σ) c | Acceptor transverse location | Acceptor orientation (θmax, σ) c | Calculated <κ2> d |
|---|---|---|---|---|---|---|---|
| t-PnA | DPH | [24,25] | 0.92 nm [26]e | 20°, 0.15 f | 0.75 nm [27]f | 0, 0.35 [27]f | 0.58 ± 0.01 |
| DPH | NBD | [28–31] | 0.75 nm [27]f | 0, 0.35 [27]f | 1.35 nm [20,32,33]e,f | 125°, 0.35 [20]f | 0.66 ± 0.01 |
| NBD | NBD | [34,35] | 1.35 nm [20,32,33]f | 125°, 0.35 [20]f | 1.35 nm [20,32,33]e,f | 0.66 ± 0.03 | |
| NBD | Carbocyanine | [36] | 1.26 nm [37]f | 77°, 0.25 [37]f | 0.76 ± 0.02 | ||
| NBD | Rhodamine B | [25,35,38–42] | 2.1 nm [43,44]f | 90°, 0.275 [43,45]g | 0.56 ± 0.02 | ||
| BODIPY | Rhodamine B | [30,46] | 0.8 nm [47]f | 10°, 0.5 [47]f | 0.57 ± 0.01 | ||
| BODIPY | Carbocyanine | [48] | 1.26 nm [37]f | 77°, 0.25 [37]f | 0.72 ± 0.02 | ||
| Rhodamine B | Rhodamine B | [25,35,41,49,50] | 2.1 nm [43,44]f | 90°, 0.275 [43,45]g | 2.1 nm [43,44]f | 90°, 0.275 [43,45]g | 1.08 ± 0.01 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Loura, L.M.S. Simple Estimation of Förster Resonance Energy Transfer (FRET) Orientation Factor Distribution in Membranes. Int. J. Mol. Sci. 2012, 13, 15252-15270. https://doi.org/10.3390/ijms131115252
Loura LMS. Simple Estimation of Förster Resonance Energy Transfer (FRET) Orientation Factor Distribution in Membranes. International Journal of Molecular Sciences. 2012; 13(11):15252-15270. https://doi.org/10.3390/ijms131115252
Chicago/Turabian StyleLoura, Luís M. S. 2012. "Simple Estimation of Förster Resonance Energy Transfer (FRET) Orientation Factor Distribution in Membranes" International Journal of Molecular Sciences 13, no. 11: 15252-15270. https://doi.org/10.3390/ijms131115252




