Alterations in Glutathione Redox Metabolism, Oxidative Stress, and Mitochondrial Function in the Left Ventricle of Elderly Zucker Diabetic Fatty Rat Heart
Abstract
:1. Introduction
2. Results and Discussion
2.1. Alterations in Oxidative Stress in Zucker Diabetic Rat Hearts
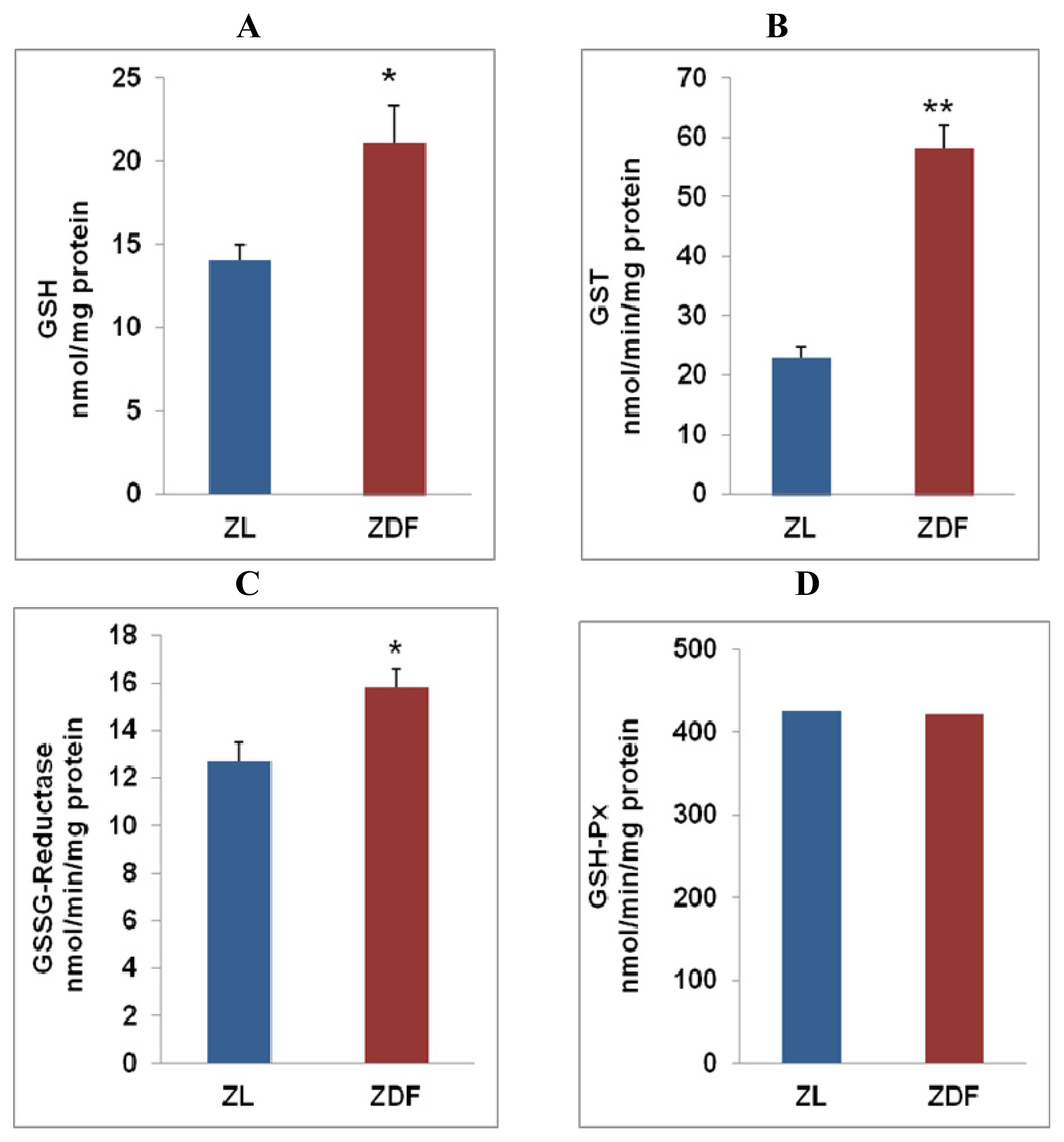
2.2. Alterations in GSH Metabolism in Zucker Diabetic Rat Hearts
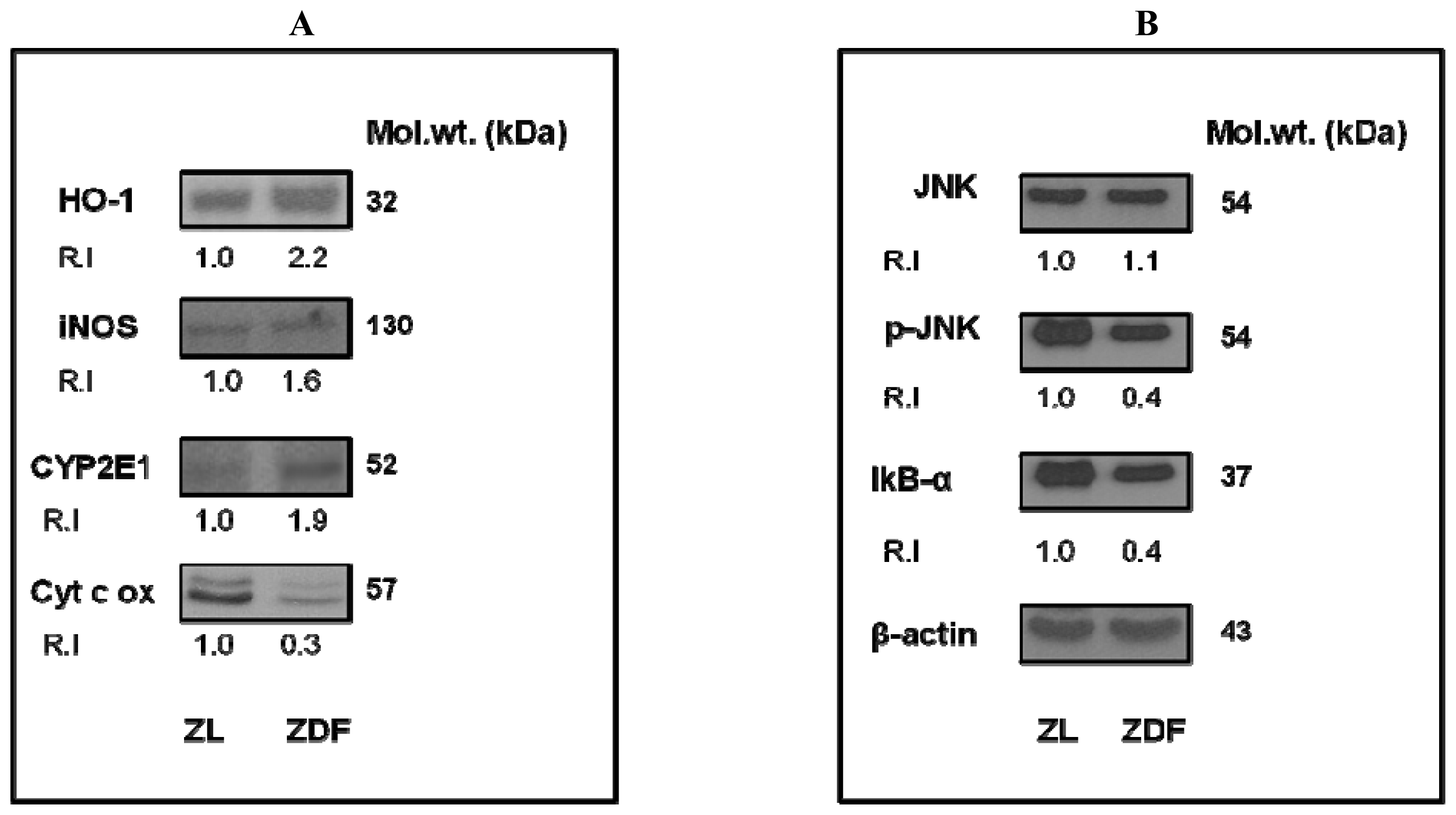
2.3. Induction of CYP450 Activity in Zucker Diabetic Rat Hearts
2.4. Alterations in Mitochondrial Respiratory Functions in ZDF Rat Hearts
2.5. Alteration in the Expression of Oxidative Stress Marker and Transcription Regulatory Proteins in ZDF Rat Hearts
2.6. Discussion
3. Experimental Section
3.1. Chemicals
3.2. Animal and Tissue Preparation
3.3. Measurement of ROS
3.4. Protein and Lipid Peroxidation (LPO) and Catalase Activities
3.5. Measurement of GSH-Redox Metabolism
3.6. Measurement of CYP 2E1 Activity
3.7. Measurement of Mitochondrial Respiratory Enzyme Complexes
3.8. SDS-PAGE and Western Blot Analysis
3.9. Statistical analysis
4. Conclusions
Acknowledgements
- Conflict of InterestThe authors declare no conflict of interest.
Abbreviations
| CYP4502E1 | cytochrome P450 2E1 |
| DCFDA | 2′,7′-dichlorofluorescein diacetate |
| DMNA | dimethylnitrosamine |
| GSH | glutathione |
| GST | glutathione S-transferase |
| GSH-Px | glutathione peroxidase |
| GSSG-reductase | glutathione reductase |
| Cyt c ox | cytochrome c oxidase |
| LPO | lipid peroxidation |
| ROS | reactive oxygen species |
| SDS-PAGE | sodium dodecylsulphate polyacrylamide gel electrophoresis |
| ZDF | Zucker diabetic fatty |
| ZL | Zucker lean |
References
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar]
- D’Souza, A.; Hussain, M.; Howarth, F.C.; Woods, N.M.; Bidasee, K.; Singh, J. Pathogenesis and pathophysiology of accelerated atherosclerosis in the diabetic heart. Mol. Cell Biochem 2009, 331, 89–116. [Google Scholar]
- Teixeira-lemos, E.; Nunes, S.; Teixeira, F.; Reis, F. Regular physical exercise training assists in preventing type 2 diabetes development: Focus on its antioxidant and anti-inflammatory properties. Cardiovasc. Diabetol 2011, 10, 12–20. [Google Scholar]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res 2010, 107, 1058–1070. [Google Scholar]
- Shen, G.X. Oxidative stress and diabetic cardiovascular disorders: Roles of mitochondria and NADPH oxidase. Can. J. Physiol. Pharmacol 2010, 88, 241–248. [Google Scholar]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev 2011, 7, 313–324. [Google Scholar]
- Clark, J.B.; Palmer, C.J.; Shaw, W.N. The diabetic Zucker fatty rat. Proc. Soc. Exp. Biol. Med 1983, 173, 68–75. [Google Scholar]
- Gustavsson, C.; Soga, T.; Wahlstrom, E.; Vesterlund, M.; Azimi, A.; Norstedt, G.; Tollet-Egnell, P. Sex-dependent hepatic transcript and metabolites in the development of glucose intolerance and insulin resistance in Zucker diabetic fatty rats. J. Mol. Endocrinol 2011, 47, 129–143. [Google Scholar]
- Verspohl, E.J. Novel phramcological approaches to the treatment of type 2 diabtes. Pharmacol. Rev 2012, 64, 188–237. [Google Scholar]
- Toblli, J.; Cao, G.; Rivas, C.; Munoz, M.; Giani, J.; Dominici, F.; Angerosa, M. Cardiovascular protective effects of nebivolol in Zucker diabetic fatty rats. J. Hypertens 2010, 28, 1007–1019. [Google Scholar]
- Howarth, F.C.; Jacobson, M.; Shafiullah, M.; Adeghate, E. Long-term effects of type 2 diabetes mellitus on heart rhythm in the Goto-Kakizaki rat. Exp. Physiol. 2008, 362–369. [Google Scholar]
- Howarth, F.C.; Qureshi, M.A.; Sobhy, Z.H.; Parekh, K.; Yammahi, S.R.; Adrian, T.E.; Adeghate, E. Structural lesions and changing pattern of expression of genes encoding cardiac muscle proteins are associated with ventricular myocyte dysfunction in type 2 diabetic Goto-Kakizaki rats fed a high-fat diet. Exp. Physiol 2011, 96, 765–777. [Google Scholar]
- Howarth, F.C.; Qureshi, M.A.; Hassan, Z.; Al Kury, L.T.; Isaev, D.; Parekh, K.; Yammahi, S.R.; Adrian, T.E.; Adeghate, E. Changing pattern of gene expression is associated with ventricular myocyte dysfunction and altered mechanisms of Ca2+ signalling in young type 2 Zucker diabetic fatty rat heart. Exp. Physiol 2011, 96, 325–337. [Google Scholar]
- Howarth, F.C.; Qureshi, M.A.; Hassan, Z.; Isaev, D.; Parekh, K.; John, A.; Oz, M.; Raza, H.; Adeghate, E.; Adrian, T.E. Contractility of ventricular myocytes is well preserved despite altered mechanisms of Ca2+ transport and a changing pattern of mRNA in elderly type 2 Zucker diabetic fatty rat heart. Mol. Cell. Biochem 2012, 361, 267–280. [Google Scholar]
- Raza, H.; John, A. Glutathione metabolism and oxidative stress in neonatal rat tissues from streptozotocin-induced diabetic mothers. Diabetes Metab. Res. Rev 2004, 20, 72–78. [Google Scholar]
- Raza, H.; Prabu, S.K.; Robin, M.-A.; Avadhani, N.G. Elevated mitochondrial cytochrome P4502E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats. Tissue specific variations and roles in oxidative stress. Diabetes 2004, 53, 185–194. [Google Scholar]
- Raza, H.; Ahmed, I.; John, A. Tissue specific expression and immunohistochemical localization of glutathione S-transferase in streptozotocin induced diabetic rats: Modulation by Momordica charantia (karela) extract. Life Sci 2004, 74, 1503–1511. [Google Scholar]
- Raza, H.; Prabu, S.K.; John, A.; Avadhani, N.G. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int. J. Mol. Sci 2011, 12, 3133–3147. [Google Scholar]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem 1952, 195, 133–140. [Google Scholar]
- Raza, H.; Ahmed, I.; John, A.; Sharma, A.K. Modulation of xenobiotic metabolism and oxidative stress in chronic streptozotocin-induced diabetic rats fed with Momordica charantia fruit extract. J. Biochem. Mol. Toxicol 2000, 14, 131–139. [Google Scholar]
- Enriquez, A.; Leclercq, I.; Farrell, G.C.; Robertson, G. Altered expression of hepatic CYP2E1 and CYP4A in obese, diabetic ob/ob mice, and fa/fa Zucker rats. Biochem. Biophys. Res. Commun 1999, 255, 300–306. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar]
- Daniels, A.; Linz, D.; van Bilsen, M.; Rutten, H.; Sadowski, T.; Ruf, S.; Juretschke, H.P.; Neumann-Haefelin, C.; Munts, C.; van der Vusse, G.J.; et al. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur. J. Heart Fail 2012, 14, 193–201. [Google Scholar]
- Wei, M.; Ong, L.; Smith, M.T.; Ross, F.B.; Schmid, K.; Hoey, A.J.; Burstow, D.; Brown, L. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ. 2003, 12, 44–50. [Google Scholar] [Green Version]
- Howarth, F.C.; Jacobson, M.; Shafiullah, M.; Adeghate, E. Long-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp. Physiol 2005, 90, 827–835. [Google Scholar]
- Shimabukuro, M.; Zhou, Y.T.; Levi, M.; Unger, R.H. Fatty acid-induced beta cell apoptosis: A link between obesity and diabetes. Proc. Natl. Acad. Sci. USA 1998, 95, 2498–2502. [Google Scholar]
- Tokuyama, Y.; Sturis, J.; DePaoli, A.M.; Takeda, J.; Stoffel, M.; Tang, J.; Sun, X.; Polonsky, K.S.; Bell, G.I. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes 1995, 44, 1447–1457. [Google Scholar]
- Wang, P.; Chatham, J.C. Onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function after ischemia in the isolated perfused heart. Am. J. Physiol. Endocrinol. MeTable 2004, 286, E725–E736. [Google Scholar]
- Serpillon, S.; Floyd, B.C.; Gupte, R.S.; George, S.; Kozicky, M.; Neito, V.; Recchia, F.; Stanley, W.; Wolin, M.S.; Gupte, S.A. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am. J. Physiol. Heart Circ. Physiol 2009, 297, H153–H162. [Google Scholar]
- Tianzheng, Y.; James, L.R.; Yisang, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar]
- Chentouf, M.; Dubois, G.; Jahannaut, C.; Castex, F.; Lajoix, A.D.; Gross, R.; Peraldi-Roux, S. Excessive food intake, obesity and inflammation process in Zucker fa/fa rat pancreatic islets. PLoS One 2011, 6, e22954. [Google Scholar]
- Lenaers, E.; de Feyter, H.M.; Hoeks, J.; Schrauwen, P.; Schaart, G.; Nabben, M.; Nicolay, K.; Prompers, J.J.; Hesselink, M.K. Adaptations in mitochondrial function parallel, but fail to rescue, the transition to severe hyperinsulinemia: A study in Zucker diabetic fatty rats. Obesity 2010, 18, 1100–1107. [Google Scholar]
- McClung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar]
- Satoretto, J.L.; Kalwa, H.; Pluth, M.D.; Lippard, S.J.; Michel, T. Hydrogen peroxide differentially modulates cardiac myocyte nitic oxide synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15792–15797. [Google Scholar]
- Zhang, W.; Lu, D.; Dong, W.; Zhang, L.; Zhang, X.; Quan, X.; Ma, C.; Lain, H.; Zhang, L. Expression of CYP2E1 increases oxidative stress and induces apoptosis of cardiomyocytes in transgenic mice. FEBS J 2011, 278, 1484–1492. [Google Scholar]
- Madian, A.G.; Myracle, A.D.; Diaz-Maldonado, N.; Rochelle, N.S.; Janle, E.M.; Regnier, F.E. Determining the effects of antioxidants on oxidative stress induced carbonylation of proteins. Anal. Chem 2011, 83, 9328–9336. [Google Scholar]
- Lakshmanan, A.P.; Harima, M.; Sukumaran, V.; Soetikno, V.; Thandavarayan, R.A.; Suzuki, K.; Kodama, M.; Nagata, M.; Takagi, R.; Watanabe, K. Modulation of AT-1R/AMPK-MAPK cascade plays crucial role for the pathogenesis of diabetic cardiomyopathy in transgenic type 2 diabetic (Spontaneous Diabetic Torii) rats. Biochem. Pharm 2012, 83, 653–660. [Google Scholar]
- Tsai, K.H.; Wang, W.J.; Lin, C.W.; Pai, P.; Lai, T.Y.; Tsai, C.Y.; Kuo, W.W. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-κB in cardiomyocytes exposed to high glucose. J. Cell. Physiol 2012, 227, 1347–1357. [Google Scholar]
- Anning, L. Activation of the JNK signaling pathway: Breaking the brake on apoptosis. BioEssays 2002, 25, 17–24. [Google Scholar]
- Syed, I.; Kyathanahalli, C.N.; Jayaram, B.; Govind, S.; Rhodes, C.J.; Kowluru, R.A.; Kowluru, A. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: Role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 2011, 60, 2843–2852. [Google Scholar]
- Aljada, A.; Mohanty, P.; Ghanim, H.; Abdo, T.; Tripathy, D.; Chaudhuri, A.; Dandona, P. increase in intranuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: Evidence for a proinflammatory effect. Am. J. Clin. Nutr 2004, 79, 682–690. [Google Scholar]
- Tang, T.; Zhang, J.; Yin, J.; Statszkiewicz, J.; Gawronska-Kozak, B.; Jung, D.Y.; Ko, H.J.; Ong, H.; Kim, J.K.; Mynatt, R.; et al. Uncoupling of inflammation and insulin resistance by NF-κB in transgenic mice through elevated energy expenditure. J. Biol. Chem 2010, 285, 4637–4644. [Google Scholar]
- Aragno, M.; Mastrocola, R.; Medana, C.; Catalano, M.G.; Vercellinatto, I.; Danni, O.; Bocuzzi, G. Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology 2006, 147, 5967–5974. [Google Scholar]



© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Raza, H.; John, A.; Howarth, F.C. Alterations in Glutathione Redox Metabolism, Oxidative Stress, and Mitochondrial Function in the Left Ventricle of Elderly Zucker Diabetic Fatty Rat Heart. Int. J. Mol. Sci. 2012, 13, 16241-16254. https://doi.org/10.3390/ijms131216241
Raza H, John A, Howarth FC. Alterations in Glutathione Redox Metabolism, Oxidative Stress, and Mitochondrial Function in the Left Ventricle of Elderly Zucker Diabetic Fatty Rat Heart. International Journal of Molecular Sciences. 2012; 13(12):16241-16254. https://doi.org/10.3390/ijms131216241
Chicago/Turabian StyleRaza, Haider, Annie John, and Frank C. Howarth. 2012. "Alterations in Glutathione Redox Metabolism, Oxidative Stress, and Mitochondrial Function in the Left Ventricle of Elderly Zucker Diabetic Fatty Rat Heart" International Journal of Molecular Sciences 13, no. 12: 16241-16254. https://doi.org/10.3390/ijms131216241



