Molecular Diversity Assessment Using Sequence Related Amplified Polymorphism (SRAP) Markers in Vicia faba L.
Abstract
:1. Introduction
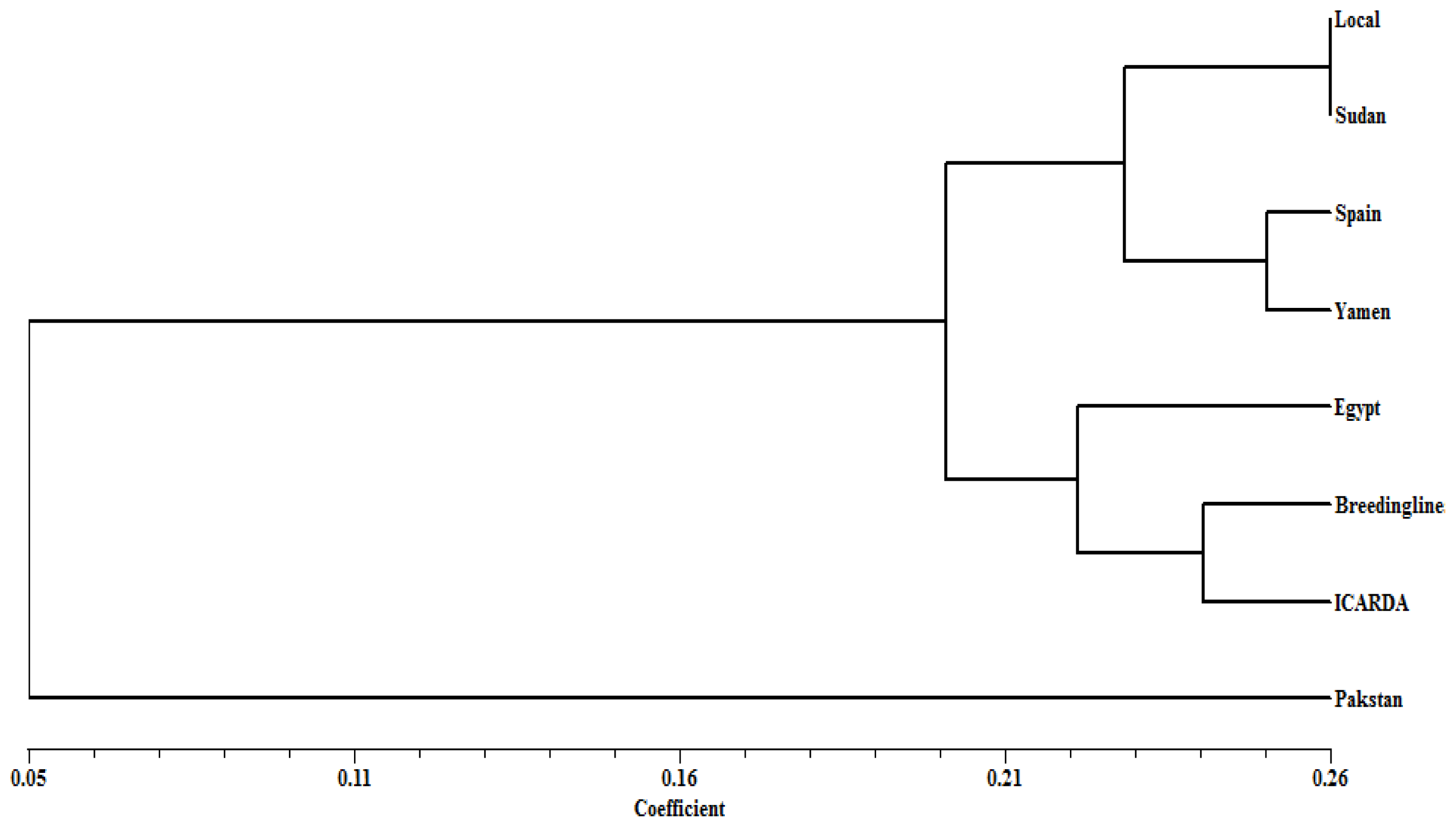
2. Results
3. Discussion
4. Experimental Section
4.1. Plant Materials and DNA Extraction
4.2. SRAP-PCR
4.3. Data Scoring and Statistical Analysis
5. Conclusions
Acknowledgements
References
- Ellwood, S.R.; Phan, H.T.; Jordan, M.; Hane, J.; Torres, A.M.; Avila, C.M.; Cruz-Izquierdo, S.; Oliver, R.P. Construction of a comparative genetic map in faba bean (Vicia faba L.); Conservation of genome structure with Lens culinaris. BMC Genomics 2008, 9, 380. [Google Scholar]
- Rispail, N.; Kalo, P.; Kiss, G.B.; Ellis, T.H.; Gallardo, K.; Thompson, R.D.; Prats, E.; Larrainzar, E.; Ladrera, R.; Gonzalez, E.M.; Esther, M.; et al. Model legumes contribute to faba bean breeding. Field Crops Res 2010, 115, 253–269. [Google Scholar]
- Link, W.; Dixkens, C.; Singh, M.; Schwall, M.; Melchinger, A.E. Genetic diversity in European and Mediterranean faba bean germplasm revealed by RAPD markers. Theor. Appl. Genet 1995, 90, 27–32. [Google Scholar]
- Avila, C.M.; Sillero, J.C.; Rubiales, D.; Moreno, M.T.; Torres, A.M. Identification of RAPD markers linked to the Uvf-1 gene conferring hypersensitive resistance against rust (Uromyces viciae-fabae) in Vicia faba L. Theor. Appl. Genet 2003, 107, 353–358. [Google Scholar]
- Zeid, M.A.; Schön, C.C.; Link, W. Genetic diversity in recent elite faba bean lines using AFLP markers. Theor. Appl. Genet 2003, 107, 1304–1314. [Google Scholar]
- Roman, B.L.; Satovic, Z.; Pozarkova, D.D.; Macas, J.; Dolezel, J.; Cubero, J.I.; Torres, A.M. Development of a composite map in Vicia faba L., breeding applications and future prospects. Theor. Appl. Genet 2004, 108, 1079–1088. [Google Scholar]
- Gutierrez, N.; Avila, C.M.; Rodriguez-Suarez, C.; Moreno, M.T.; Torres, A.M. Development of SCAR markers linked to a gene controlling absence of tannins in faba bean. Mol. Breed 2007, 19, 305–314. [Google Scholar]
- Van de Ven, W.T.; Powell, W.; Ramsay, G.; Waugh, R. Restriction fragment length polymorphisms as genetic markers in Vicia. Heredity 1990, 65, 329–342. [Google Scholar]
- Torres, A.M.; Weeden, N.F.; Martin, A. Linkage among isozyme, RFLP and RAPD markers in Vicia faba L. Theor. Appl. Genet 1993, 85, 937–945. [Google Scholar]
- Alghamdi, S.S. Varietal identification and genetic purity assessment of F1 hybrid seeds using RAPD markers in faba bean (Vicia faba L.). Acta Hortic 2009, 829, 269–274. [Google Scholar]
- Zong, X.; Liu, X.Z.; Guan, J.P.; Wang, S.; Liu, Q.; Paull, J.G.; Redden, R.J. Molecular variation among Chinese and global winter faba bean germplasm. Theor. Appl. Genet 2009, 118, 971–978. [Google Scholar]
- Zong, X.; Ren, J.; Guan, J.P.; Wang, S.; Liu, Q.; Paull, J.G.; Redden, R.J. Molecular variation among Chinese and global germplasm in spring faba bean areas. Plant Breed 2010, 129, 508–513. [Google Scholar]
- Terzopoulos, P.J.; Bebeli, P.J. Genetic diversity analysis of Mediterranean faba bean (Vicia faba L.) with ISSR markers. Field Crops Res 2008, 108, 39–44. [Google Scholar]
- Alghamdi, S.S.; Al-Faifi, S.A.; Migdadi, H.M.; Ammar, M.H.; Siddique, K.M. Inter-simple sequence repeat (ISSR)-based diversity assessment among faba bean genotypes. Crop Pasture Sci 2011, 62, 755–760. [Google Scholar]
- Duc, G.; Bao, S.Y.; Baum, M.; Redden, B.; Sadiki, M.; Suso, M.J.; Vishniakova, M.; Zong, X.X. Diversity maintenance and use of Vicia faba L. genetic resources. Field Crops Res 2010, 115, 270–278. [Google Scholar]
- Kwon, S.J.; Jinguo, H.; Clarice, J.C. Genetic diversity and relationship among faba bean (Vicia faba L.) germplasm entries as revealed by TRAP markers. Plant Genet. Resour 2010, 8, 204–213. [Google Scholar]
- Van de Ven, W.T.; Duncan, N.; Ramsay, G.; Phillips, M.S.; Powell, W.; Waugh, R. Taxonomic relationships between V. faba L. and its relatives based on nuclear and mitochondrial RFLPs and PCR analysis. Theor. Appl. Genet 1993, 86, 71–80. [Google Scholar]
- Shiran, B.; Mashayekh, A.M. Evaluation of the chlroplast DNA among Vicia faba L. germplasm using restriction-site analysis. Iranian J. Sci. Tech. Transac. A 2004, 28, 51–55. [Google Scholar]
- Flamand, M.C.; Goblet, J.P.; Duc, G.; Briquet, M.; Boutry, M. Sequence and transcription analysis of mitochondrial plasmids isolated from cytoplasmic male-sterile lines of Vicia faba L. Plant Mol. Biol 1992, 19, 913–923. [Google Scholar]
- Flamand, M.C.; Due, G.; Goblet, J.P.; Hong, L.; Louis, O.; Briquet, M.; Boutry, M. Variant mitochondrial plasmids of broad bean arose by recombination and are controlled by the nuclear genome. Nucl. Acids Res 1993, 21, 5468–5473. [Google Scholar]
- Sanz, A.M.; Gonzalez, S.G.; Syed, N.H.; Suso, M.J.; Saldaña, C.C.; Flavell, A.J. Genetic diversity analysis in Vicia species using retrotransposon-based SSAP markers. Mol. Genet. Genom 2007, 278, 433–441. [Google Scholar]
- Li, G.Y.; Quiros, C.F. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction, its application to mapping and gene tagging in Brassica. Theor. Appl. Genet 2001, 103, 455–461. [Google Scholar]
- Rana, M.; Singh, S.P.; Bhat, K. Fingerprinting Indian lentil (Lens culinaris ssp. culinaris Medik.) Cultivars and Landraces for Diversity Analysis Using Sequence-Related Amplified Polymorphism (SRAP) Markers. Proceedings of Fourth International Food and Legumes Research Conference, New Delhi, India; 2009; pp. 617–624. [Google Scholar]
- Vandemark, G.J.; Ariss, J.J.; Bauchan, G.A.; Larsen, R.C.; Hughes, T.J. Estimating genetic relationships among historical sources of alfalfa germplasm and selected cultivars with sequence related amplified polymorphisms. Euphytica 2006, 152, 9–16. [Google Scholar]
- Ariss, J.J.; Vandemark, G.J. Assessment of genetic diversity among nondormant and semidormant alfalfa populations using sequence-related amplified polymorphisms. Crop Sci 2007, 47, 2274–2284. [Google Scholar]
- Castonguay, Y.; Cloutier, J.; Bertrand, A.; Michaud, R.; Laberge, S. SRAP polymorphisms associated with superior freezing tolerance in alfalfa (Medicago sativa spp. sativa). Theor. Appl. Genet 2010, 120, 1611–1619. [Google Scholar]
- Esposito, M.A.; Martin, E.A.; Craverom, V.P.; Cointry, E. Characterization of pea accessions by SRAP’s markers. Sci. Hort 2007, 113, 329–335. [Google Scholar]
- Al-Ali, A.A.; Alghamdi, S.S.; Alfifi, S.A. Evaluation of Morphological and Molecular Variations among Faba Bean Genotypes Differing in Their Drought Tolerance Levels. Proceedings of 5th International Food Legumes Research Conference (IFLRC V), Antalya, Turkey, 26–30 April 2010.
- Gong, Y.M.; Xu, S.C.; Mao, W.H.; Li, Z.Y.; Hu, Q.Z.; Zhang, G.W.; Ding, J. Genetic diversity analysis of faba bean (Vicia faba L.) based on EST-SSR markers. Agric. Sci. China 2010, 10, 838–844. [Google Scholar]
- Požárková, D.; Koblížková, A.; Román, B.; Torres, A.M.; Lucretti, S.; Lysak, M.; Doležel, J.; Macas, J. Development and characterization of microsatellite markers from chromosome 1-specific DNA libraries of Vicia faba L. Biol. Plantarum 2002, 45, 337–345. [Google Scholar]
- Zeid, M.A.; Mitchell, S.E.; Link, W.; Carter, M.; Nawar, A.I.; Fulton, T.; Kresovich, S. Simple sequence repeats (SSRs) in faba bean, new loci from Orobanche-resistant cultivar ‘Giza 402’. Plant Breed 2009, 128, 149–155. [Google Scholar]
- Torres, A.M.; Avila, C.M.; Gutierrez, N.; Palomino, C.; Moreno, M.T.; Cubero, J.I. Marker-assisted selection in faba bean (Vicia faba L.). Field Crops Res 2010, 115, 243–252. [Google Scholar]
- Dudley, J.W. Comparison of Genetic Distance Estimators Using Molecular Marker Data. Proceedings of the Symposium on Analysis of Molecular Marker Data, Corvallis, OR, USA, 5–6 August 1994; pp. 3–7.
- Pejic, I.; Ajmone-Marsan, P.; Morgante, M.; Kozumplick, V.; Castiglioni, P.; Taramino, G.; Motto, M. Comparative analysis of genetic similarity among maize inbred lines detected by RFLPs, RAPDs, SSRs and AFLPs. Theor. Appl. Genet 1998, 97, 1248–1255. [Google Scholar]
- Ferriol, M.; Pico, B.; Fernandez, P.; Cordova, D.; Nuez, F. Molecular diversity of a germplasm collection of squash (Cucurbita moschata) determined by SRAP and AFLP markers. Crop Sci 2004, 44, 653–664. [Google Scholar]
- Budak, H.; Shearman, R.C.; Parmaksiz, I.; Gaussoin, R.E.; Riordan, T.P.; Dweikat, I. Molecular characterization of buffalograss germplasm using sequence-related amplified polymorphism markers. Theor. Appl. Genet 2004, 108, 328–334. [Google Scholar]
- Suman, A.; Kimbeng, C.A.; Edmé, S.J.; Veremis, J.C. Sequence-related amplified polymorphism (SRAP) markers for assessing genetic relationships and diversity in sugarcane germplasm collections. Plant Genet. Resour 2008, 6, 222–231. [Google Scholar]
- Dong, P.; Wei, Y.M.; Chen, G.Y.; Li, W.H.; Wang, J.R.; Nevo, E.; Zheng, Y.L. Sequence-related amplified polymorphism (SRAP) of wild emmer wheat (Triticum dicoccoides) in Israel and its ecological association. Biochem. Syst. Ecol 2010, 38, 1–11. [Google Scholar]
- Zhang, Y.N.; Sun, S.J.; Zhang, X.C.; Wang, L.S.; Che, Z. Analysis on genetic diversity and genetic basis of the main sesame cultivars released in China. Agric. Sci. China 2011, 10, 509–518. [Google Scholar]
- Zhang, Y.N.; Zhang, X.C.; Hua, W.; Wang, L.S.; Che, Z. Analysis of genetic diversity among indigenous landraces from sesame (Sesamum indicum L.) core collection in China as revealed by SRAP and SSR markers. Genes Genom 2010, 32, 207–215. [Google Scholar]
- Peng, S.; Feng, N.; Guo, M.; Chen, G.Y.; Guo, Q.S. Genetic variation of Carthamus tinctorius L. and related species revealed by SRAP analysis. Biochem. Syst. Ecol 2008, 36, 531–538. [Google Scholar]
- Talebi, M.B.; Mokhtari, N.; Rahimmalek, M.; Sahhafi, S. Molecular characterization of Carthamus tinctorius and C. oxyacanthus germplasm using sequence related amplified polymorphism (SRAP) markers. Plant Omics J 2012, 5, 136–142. [Google Scholar]
- Rana, M.; Arora, K.; Singh, S.P.; Singh, A.K. Multi-locus DNA fingerprinting and genetic diversity in jute (Corchorus spp.) based on sequence-related amplified polymorphism. J. Plant Biochem. Biotechnol 2012. [Google Scholar] [CrossRef]
- Talebi, M.B.; Hajiahmadi, Z.; Rahimmalek, M. Genetic diversity and population structure of four Iranian alfalfa populations revealed by sequence-related amplified polymorphism (SRAP) Markers. J. Crop Sci. Biotech 2011, 14, 173–178. [Google Scholar]
- Mishra, M.K.; Suresh, N.; Bhat, A.M.; Suryaprakash, N.; Kumar, S.S.; Kumar, A.; Jayarama. Genetic molecular analysis of Coffea arabica (Rubiaceae) hybrids using SRAP markers. Rev. Biol. Trop. 2011, 59, 607–617. [Google Scholar]
- Youssef, M.; James, A.C.; Rivera-Madrid, R.; Ortiz, R.; Escobedo-Gracia Medrano, R.M. Musa genetic diversity revealed by SRAP and AFLP. Mol. Biotechnol 2011, 47, 189–199. [Google Scholar]
- Hao, Q.; Liu, Z.A.; Shu, Q.Y.; Zhang, R.; Rick, J.D.; Wang, L.S. Studies on Paeonia cultivars and hybrids identification based on SRAP analysis. Hereditas 2008, 145, 38–47. [Google Scholar]
- Lan, Q.I.; Wang, W.Q.; Zhang, Z.W.; Ye, J.Q.; Li, K.M. DNA fingerprints of 18 cassava varieties based on sequence-related amplified polymorphism markers. Acta Agron. Sin 2010, 36, 1642–1648. [Google Scholar]
- Li, H.; Chen, H.; Zhuang, T.; Chen, J. Analysis of genetic variation in eggplant and related Solanum species using sequence-related amplified polymorphism markers. Sci. Hort 2010, 125, 19–24. [Google Scholar]
- Wu, Y.G.; Guo, Q.S.; He, J.C.; Lin, Y.F.; Luo, L.J.; Liu, G.D. Genetic diversity analysis among and within populations of Pogostemon cablin from China with ISSR and SRAP markers. Biochem. Syst. Ecol 2010, 38, 63–72. [Google Scholar]
- Wu, X.Y.; Chen, B.Y.; Lu, G.; Wang, H.Z.; Xu, K.; Guizhan, G.; Song, Y. Genetic diversity in oil and vegetable mustard (Brassica juncea) landraces revealed by SRAP markers. Genet. Resour. Crop Evol 2009, 56, 1011–1022. [Google Scholar]
- Ferriol, M.; Pico, B.; Nuez, F. Genetic diversity of a germplasm collection of Cucurbita pepo using SRAP and AFLP markers. Theor. Appl. Genet 2003, 107, 271–282. [Google Scholar]
- Gulsen, O.; Karagul, S.; Abak, K. Diversity and relationships among Turkish okra germplasm by SRAP and phenotypic marker polymorphism. Biologia 2007, 62, 41–45. [Google Scholar]
- Suso, M.J.; Moreno, M.T. Variation in outcrossing rate and genetic structure on six culitvars of Vicia faba L. as affected by geographic location and year. Plant Breed 1999, 118, 347–350. [Google Scholar]
- Suso, M.J.; Pierre, J.; Moreno, M.T.; Esnault, R.; Le Guen, J. Variation in outcrossing levels in faba bean cultivars, role of ecological factors. J. Agric. Sci 2001, 136, 399–405. [Google Scholar]
- Suso, M.J.; Gilsanz, S.; Duc, G.; Marget, P.; Moreno, M.T. Germplasm management of faba bean (Vicia faba L.), monitoring intercrossing between accessions with interplot barriers. Genet. Resour. Crop Evol 2006, 53, 1427–1439. [Google Scholar]
- Suso, M.J.; Nadal, S.M.; Roman, B.L.; Gilsanz, S. Vicia faba L. germplasm multiplication, floral traits associated with pollen-mediated gene flow under diverse between-plot isolation strategies. Ann. Appl. Biol 2008, 152, 201–208. [Google Scholar]
- Gresta, F.; Avola, G.; Albertini, E.; Raggi, L.; Abbate, V. A study of variability in the Sicilian faba bean landrace ‘Larga di Leonforte’. Genet. Resour. Crop Evol 2010, 57, 523–531. [Google Scholar]
- Schill, B.; Melchinger, A.E.; Gumber, R.K.; Link, W. Comparison of intra- and inter-pool crosses in faba bean (Vicia faba L.). II. Genetic effects estimated from generation means in Mediterranean and German environments. Plant Breed 1998, 117, 351–359. [Google Scholar]
- Cubero, J.I. Faba Bean Improvement; Hawtin, G., webb, C., Eds.; Martinus nijhoff: The Hague, The Netherlands, 1982; pp. 91–108. [Google Scholar]
- Hanelt, P. Die intraspezifische Variabilität von Vicia faba L. und ihre Gliederung. Kulturpflanze 1972, 20, 75–128. [Google Scholar]
- Hoelzel, A.R. Molecular Genetic Analysis of Populations, Apractical Approach, 2nd ed; Oxford University Press: NewYork, NY, USA, 1998. [Google Scholar]
- Wooten, J.A.; Tolley-Jordan, L.R. Validation of phylogenetic signals in amplified fragment length data:testing the utility and reliability in closely related taxa. BMC Res. Notes 2009, 2, 26. [Google Scholar]
- Anderson, J.A.; Churchill, G.A.; Autrique, J.E.; Tanksley, S.D.; Sorrells, M.E. Optimizing parental selection for genetic linkage maps. Genome 1993, 36, 181–186. [Google Scholar]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat 1908, 44, 223–270. [Google Scholar]
- Rohlf, F.J. Ntsys-pc, Numerical Taxonomy and Multivariate Analysis System, Version 2.2; Exeter Publishing Ltd.: New York, NY, USA, 2005. [Google Scholar]
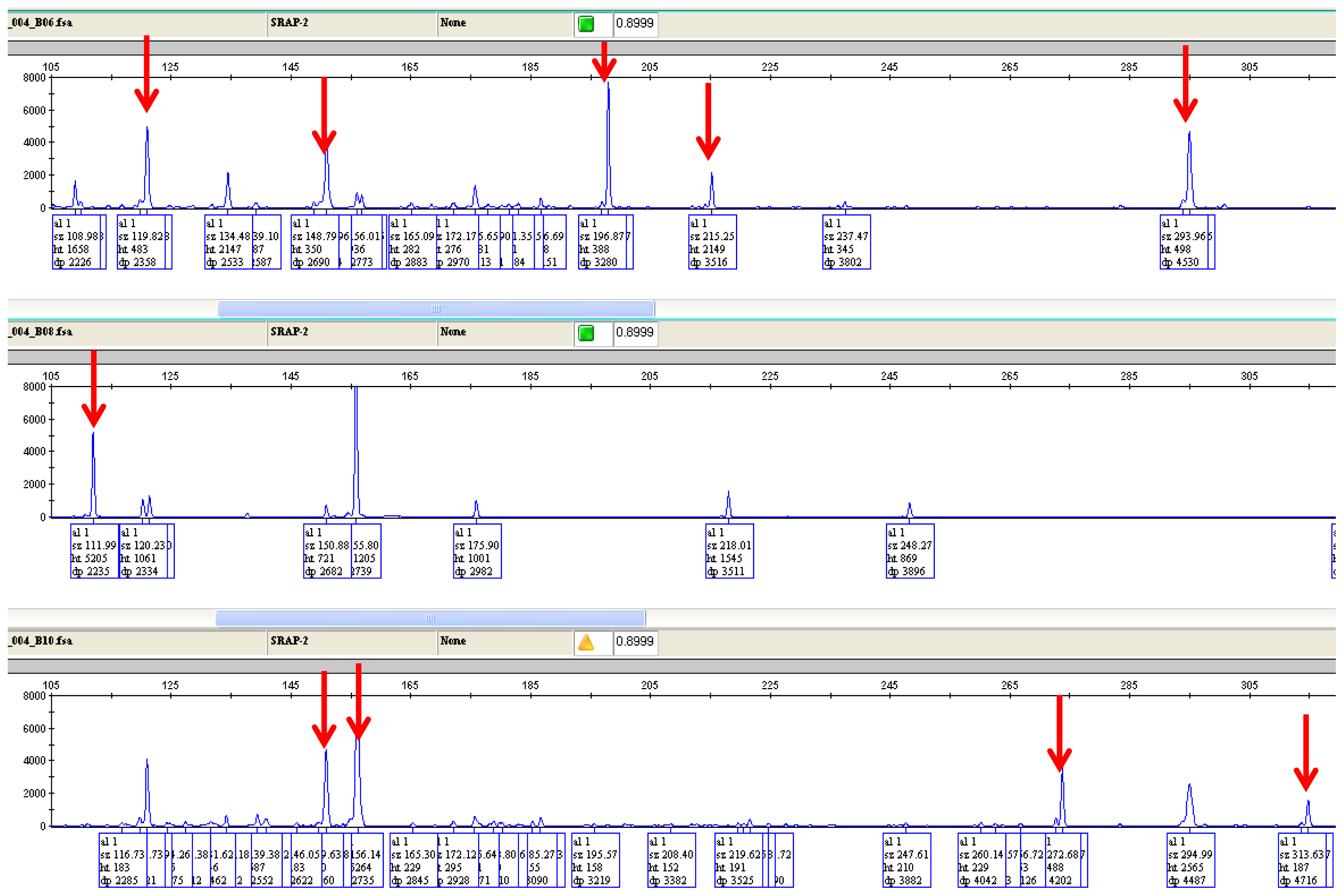
| Primer combination | Total fragments a | Average fragments b | Total no. of fragments c | PIC value |
|---|---|---|---|---|
| ME1/EM1 | 85 | 18 | 1,047 | 0.97 |
| ME1/EM2 | 158 | 26 | 1,563 | 0.99 |
| ME1/EM3 | 134 | 31 | 1,817 | 0.99 |
| ME1/EM4 | 56 | 11 | 662 | 0.97 |
| ME2/EM1 | 46 | 8 | 444 | 0.95 |
| ME2/EM2 | 108 | 24 | 1,417 | 0.98 |
| ME2/EM4 | 10 | 3 | 190 | 0.84 |
| ME3/EM1 | 77 | 17 | 1,013 | 0.97 |
| ME3/EM2 | 69 | 7 | 414 | 0.97 |
| ME3/EM3 | 66 | 5 | 323 | 0.98 |
| ME3/EM4 | 54 | 10 | 567 | 0.96 |
| ME4/EM2 | 59 | 5 | 323 | 0.97 |
| ME4/EM3 | 16 | 4 | 220 | 0.92 |
| ME4/EM4 | 98 | 12 | 700 | 0.98 |
| Total | 1,036 | - | 10,700 | - |
| Average | 74 | 13 | 764 | 0.96 |
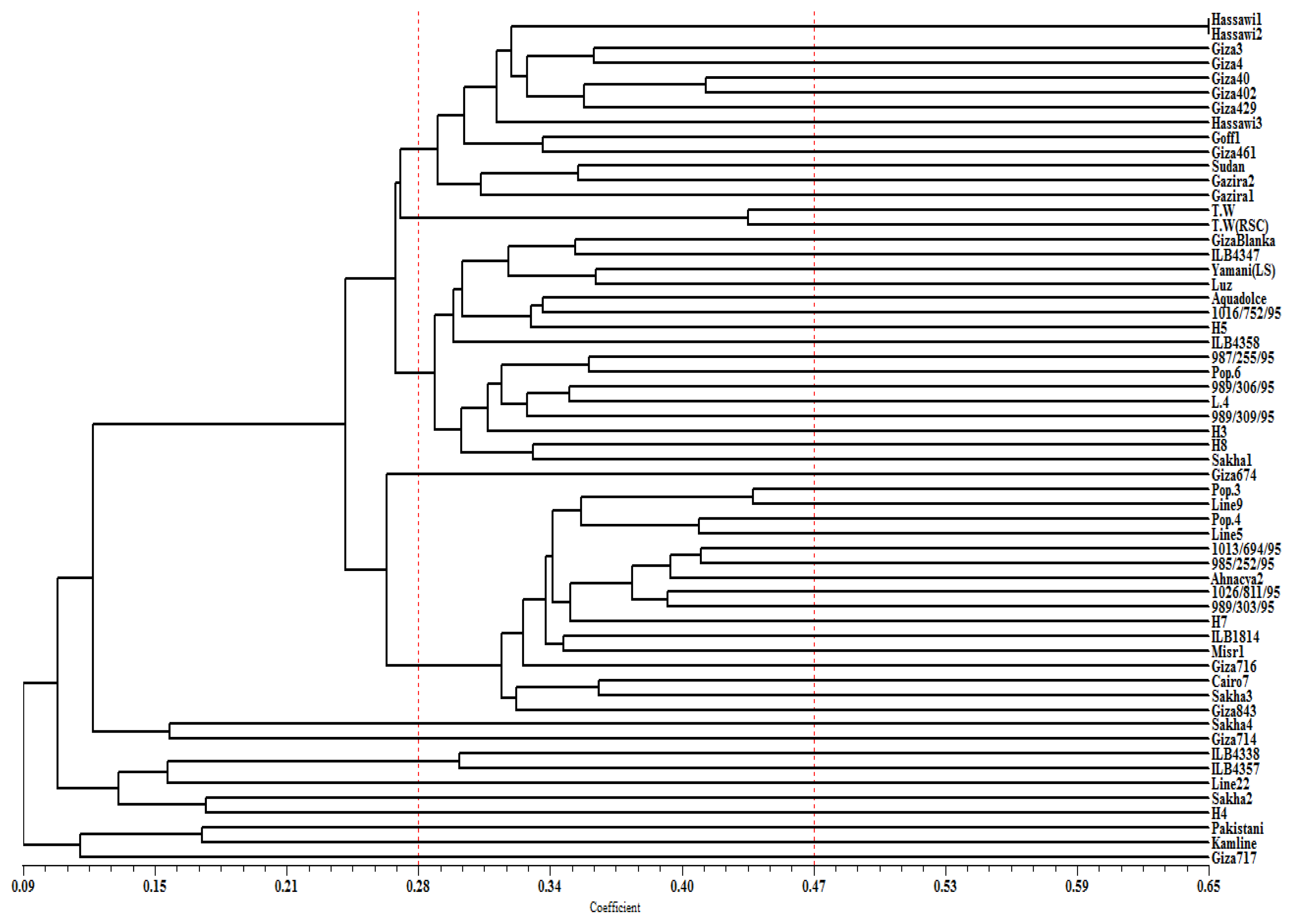
| Entry No. | Entry name | Origin | Seed type |
|---|---|---|---|
| 1 | Hassawi1 | KSA | Equine |
| 2 | Hassawi2 | KSA | Equine |
| 3 | Hassawi3 | KSA | Equine |
| 4 | Goff1 | KSA | Equine |
| 5 | T.W.(red seed) | KSA | Equine |
| 6 | H4 | KSA | Equine |
| 7 | H7 | KSA | Equine |
| 8 | Line 9 | KSA | Equine |
| 9 | Line 5 | KSA | Equine |
| 10 | Line 22 | KSA | Equine |
| 11 | Pop.6 | KSA | Equine |
| 12 | H3 | KSA | Equine |
| 13 | H5 | KSA | Equine |
| 14 | H8 | KSA | Equine |
| 15 | L. 4 | KSA | Equine |
| 16 | Pop. 3 | KSA | Equine |
| 17 | Pop. 4 | KSA | Equine |
| 18 | Giza 3 | KSA | Equine |
| 19 | Giza 4 | Egypt | Equine |
| 20 | Giza 40 | Egypt | Equine |
| 21 | Giza 402 | Egypt | Equine |
| 22 | Giza 429 | Egypt | Equine |
| 23 | Giza 461 | Egypt | Equine |
| 24 | Gizablanka | Egypt | Major |
| 25 | 1013/694/95 | Egypt | Equine |
| 26 | 1026/811/95 | Egypt | Equine |
| 27 | 985/252/95 | Egypt | Equine |
| 28 | 989/303/95 | Egypt | Equine |
| 29 | Misr 1 | Egypt | Equine |
| 30 | Sakha 2 | Egypt | Major |
| 31 | Sakha 3 | Egypt | Equine |
| 32 | Giza 716 | Egypt | Major |
| 33 | Giza 717 | Egypt | Equine |
| 34 | Giza 843 | Egypt | Equine |
| 35 | 1016/752/95 | Egypt | Equine |
| 36 | 987/255/95 | Egypt | Major |
| 37 | 989/306/95 | Egypt | Major |
| 38 | 989/309/95 | Egypt | Major |
| 39 | Sakha 1 | Egypt | Equine |
| 40 | Sakha 4 | Egypt | Equine |
| 41 | Giza 674 | Egypt | Equine |
| 42 | Giza 714 | Egypt | Equine |
| 43 | Cairo 7 | Egypt | Equine |
| 44 | ILB 4338 | ICARDA | Equine |
| 45 | ILB 4357 | ICARDA | Equine |
| 46 | ILB 1814 | ICARDA | Major |
| 47 | Ahnacya 2 | ICARDA | Equine |
| 48 | ILB 4347 | ICARDA | Equine |
| 49 | ILB 4358 | ICARDA | Major |
| 50 | Pakistani | Pakistan | Minor |
| 51 | Luz | Spain | Major |
| 52 | Aquadolce | Spain | Major |
| 53 | Kamline | Spain | Minor |
| 54 | Sudan | Sudan | Equine |
| 55 | Gazira 1 | Sudan | Major |
| 56 | Gazira 2 | Sudan | Minor |
| 57 | T.W. | Sudan | Equine |
| 58 | Yamani(Large seed) | Yemen | Major |
| Forward primers | 5′→3′ | Reverse primers | 5′→3′ |
|---|---|---|---|
| ME1 | TGAGTCCAAACCGGAA | EM1 | GACTGCGTACGAATTAAT |
| ME2 | TGAGTCCAAACCGGAC | EM2 | GACTGCGTACGAATTTGC |
| ME3 | TGAGTCCAAACCGGAT | EM3 | GACTGCGTACGAATTGAC |
| ME4 | TGAGTCCAAACCGGAC | EM4 | GACTGCGTACGAATTTGA |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alghamdi, S.S.; Al-Faifi, S.A.; Migdadi, H.M.; Khan, M.A.; EL-Harty, E.H.; Ammar, M.H. Molecular Diversity Assessment Using Sequence Related Amplified Polymorphism (SRAP) Markers in Vicia faba L.. Int. J. Mol. Sci. 2012, 13, 16457-16471. https://doi.org/10.3390/ijms131216457
Alghamdi SS, Al-Faifi SA, Migdadi HM, Khan MA, EL-Harty EH, Ammar MH. Molecular Diversity Assessment Using Sequence Related Amplified Polymorphism (SRAP) Markers in Vicia faba L.. International Journal of Molecular Sciences. 2012; 13(12):16457-16471. https://doi.org/10.3390/ijms131216457
Chicago/Turabian StyleAlghamdi, Salem S., Sulieman A. Al-Faifi, Hussein M. Migdadi, Muhammad Altaf Khan, Ehab H. EL-Harty, and Megahed H. Ammar. 2012. "Molecular Diversity Assessment Using Sequence Related Amplified Polymorphism (SRAP) Markers in Vicia faba L." International Journal of Molecular Sciences 13, no. 12: 16457-16471. https://doi.org/10.3390/ijms131216457






