High Density Lipoprotein Protects Mesenchymal Stem Cells from Oxidative Stress-Induced Apoptosis via Activation of the PI3K/Akt Pathway and Suppression of Reactive Oxygen Species
Abstract
:1. Introduction
2. Results and Discussion
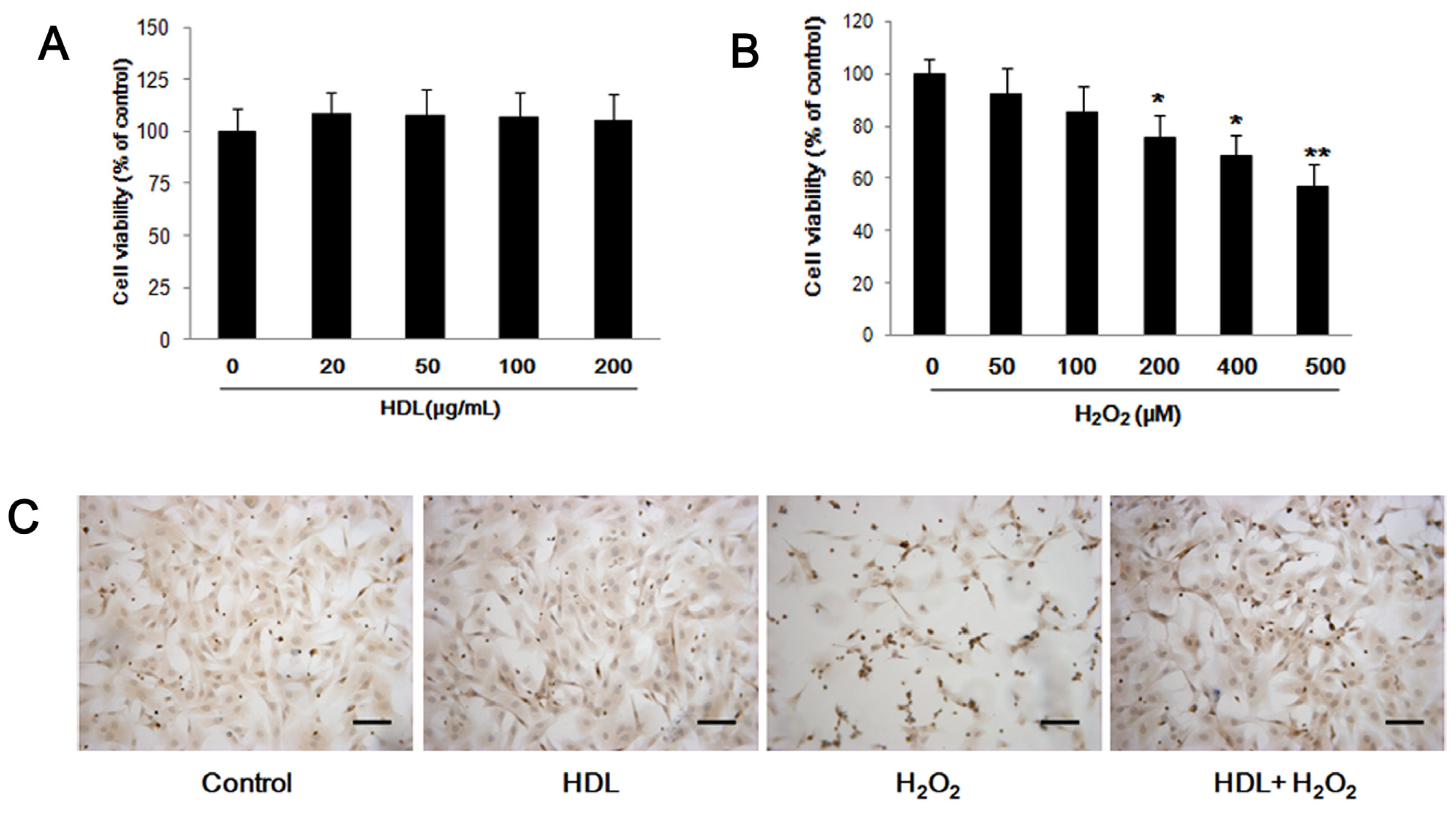
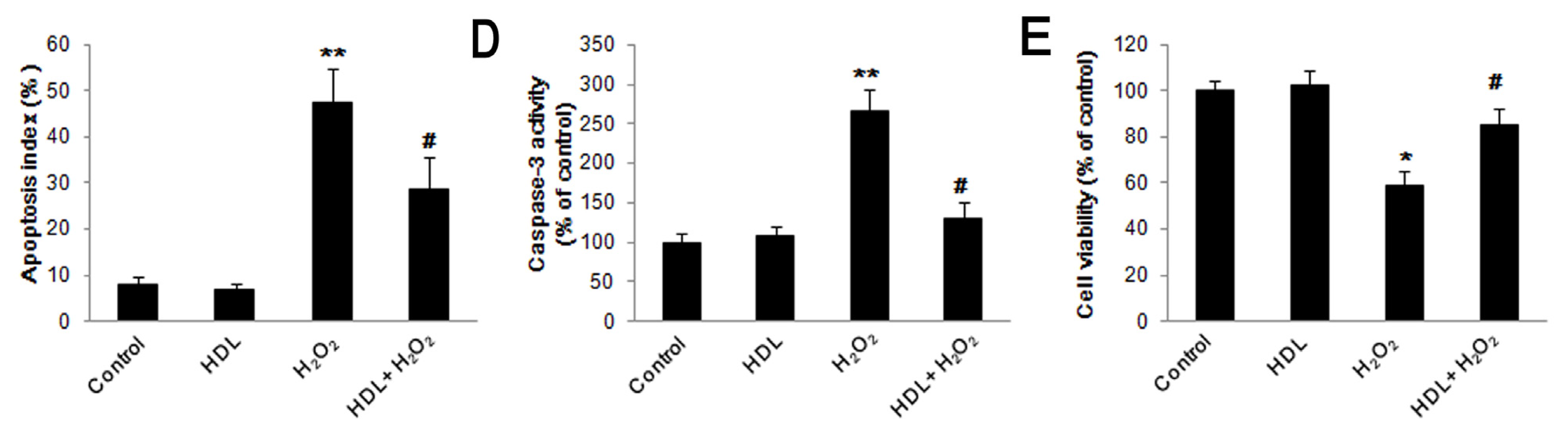
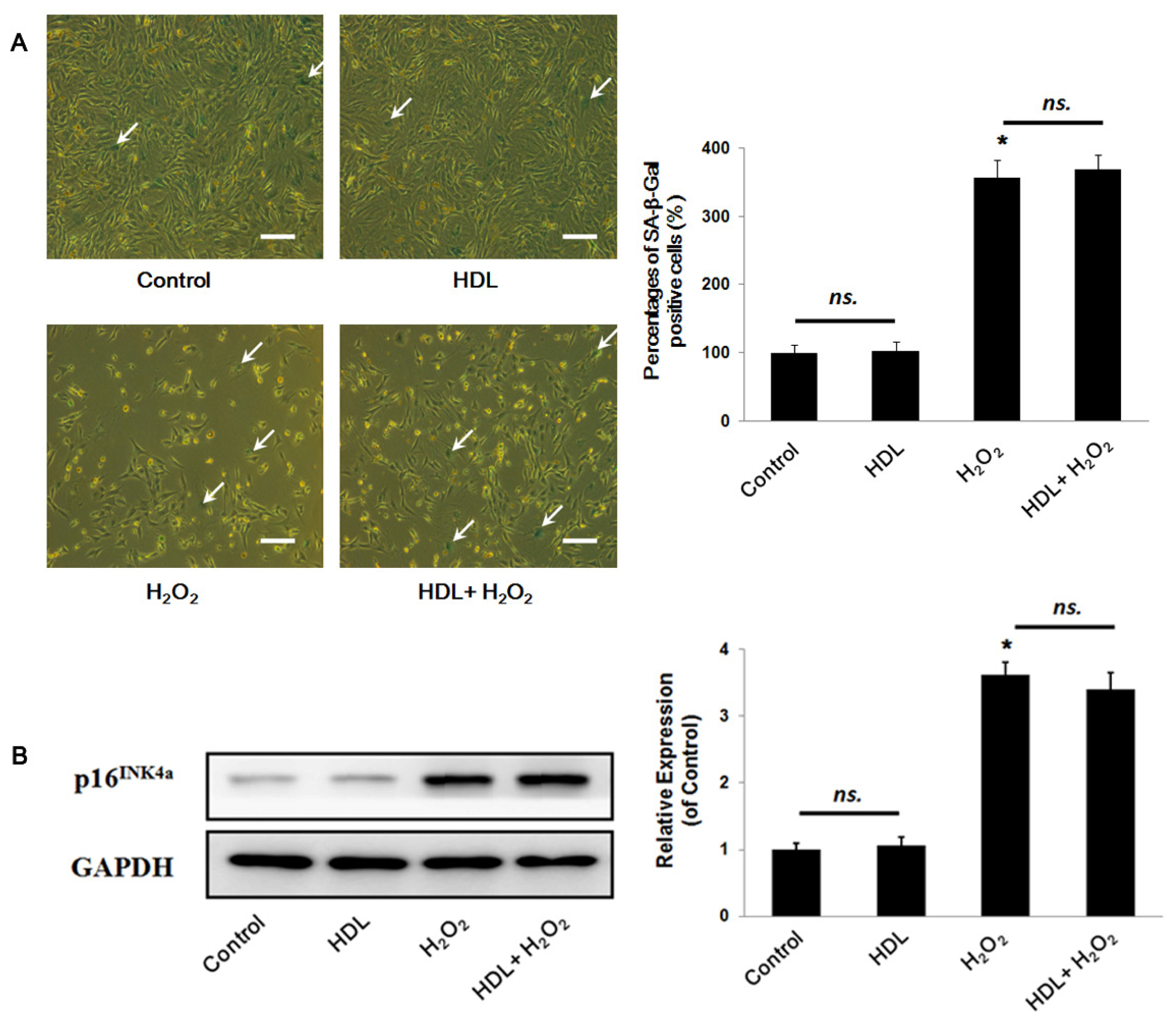
2.1. HDL Protects MSCs against H2O2-Induced Apoptosis
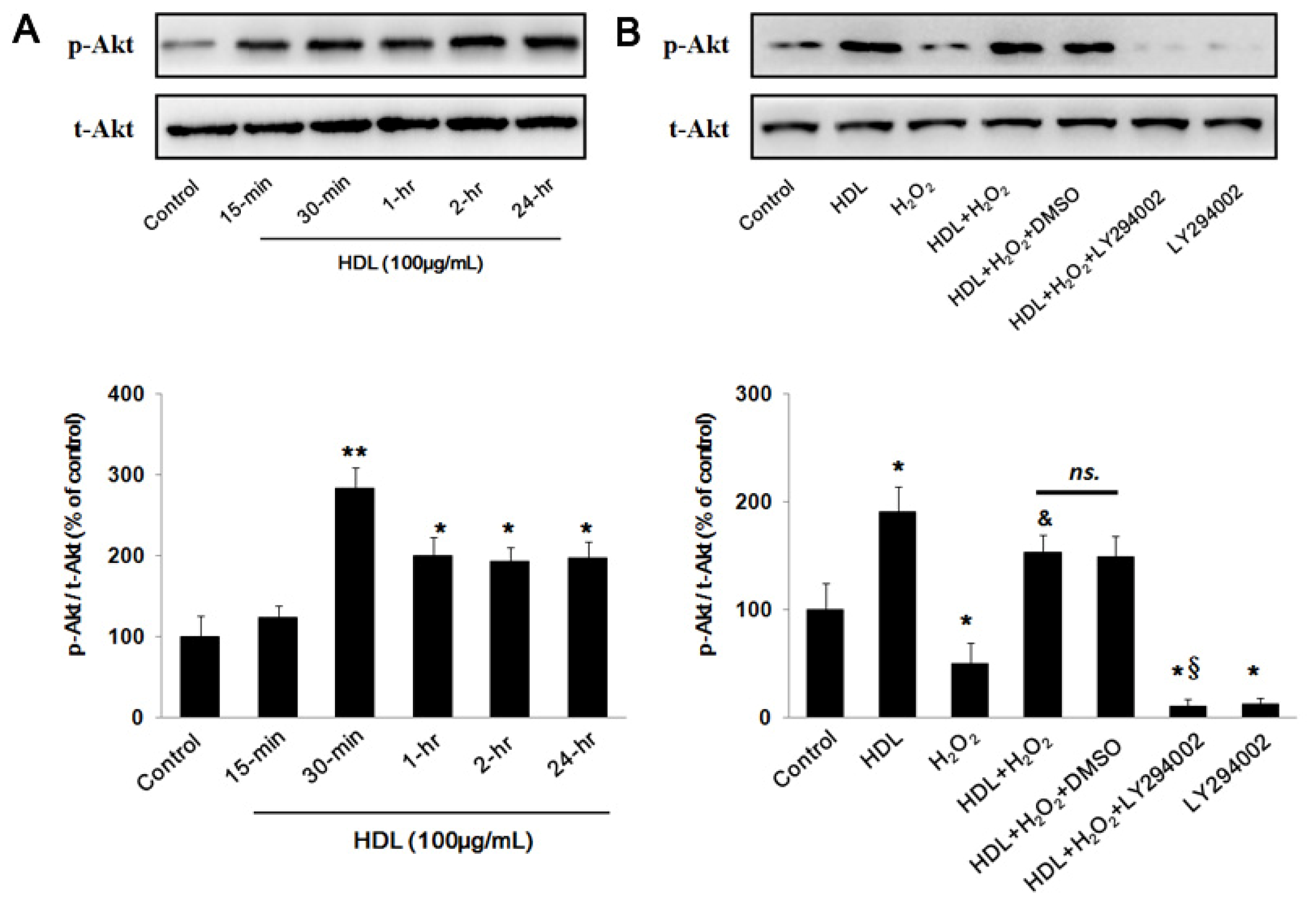
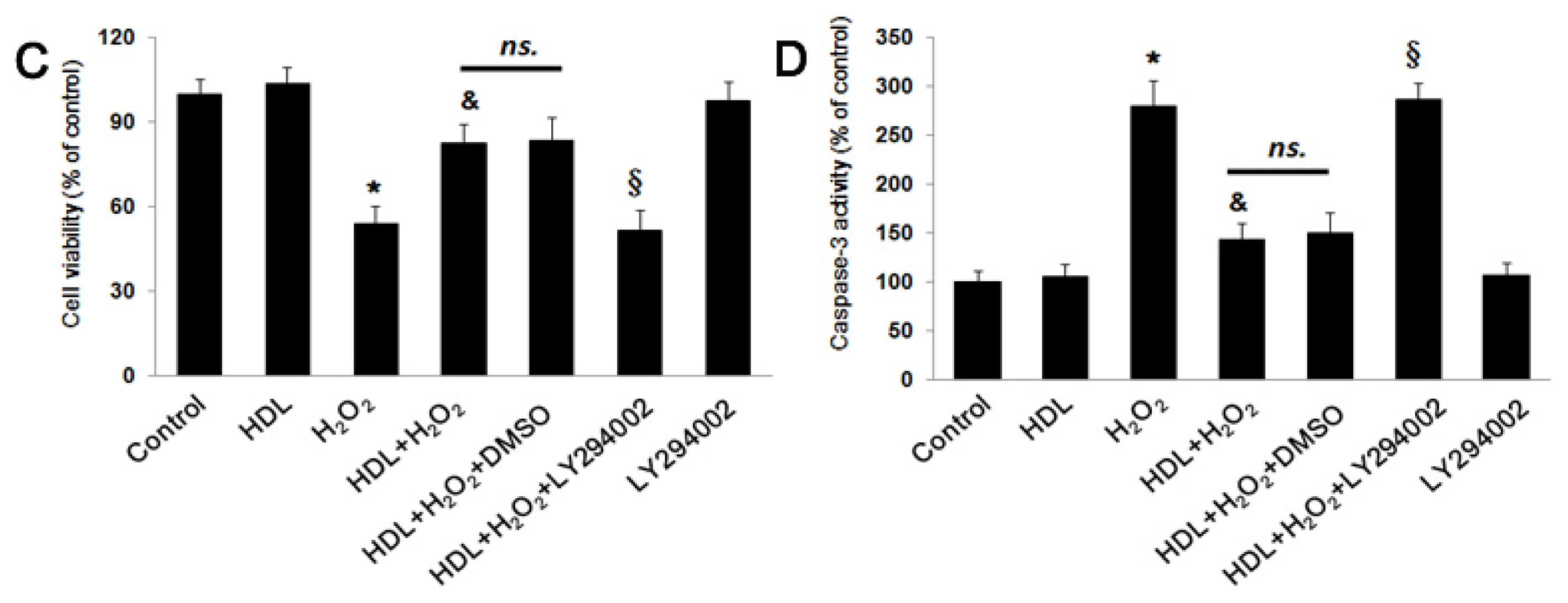
2.2. PI3K/Akt Pathway Involves in the Protective Effect of HDL
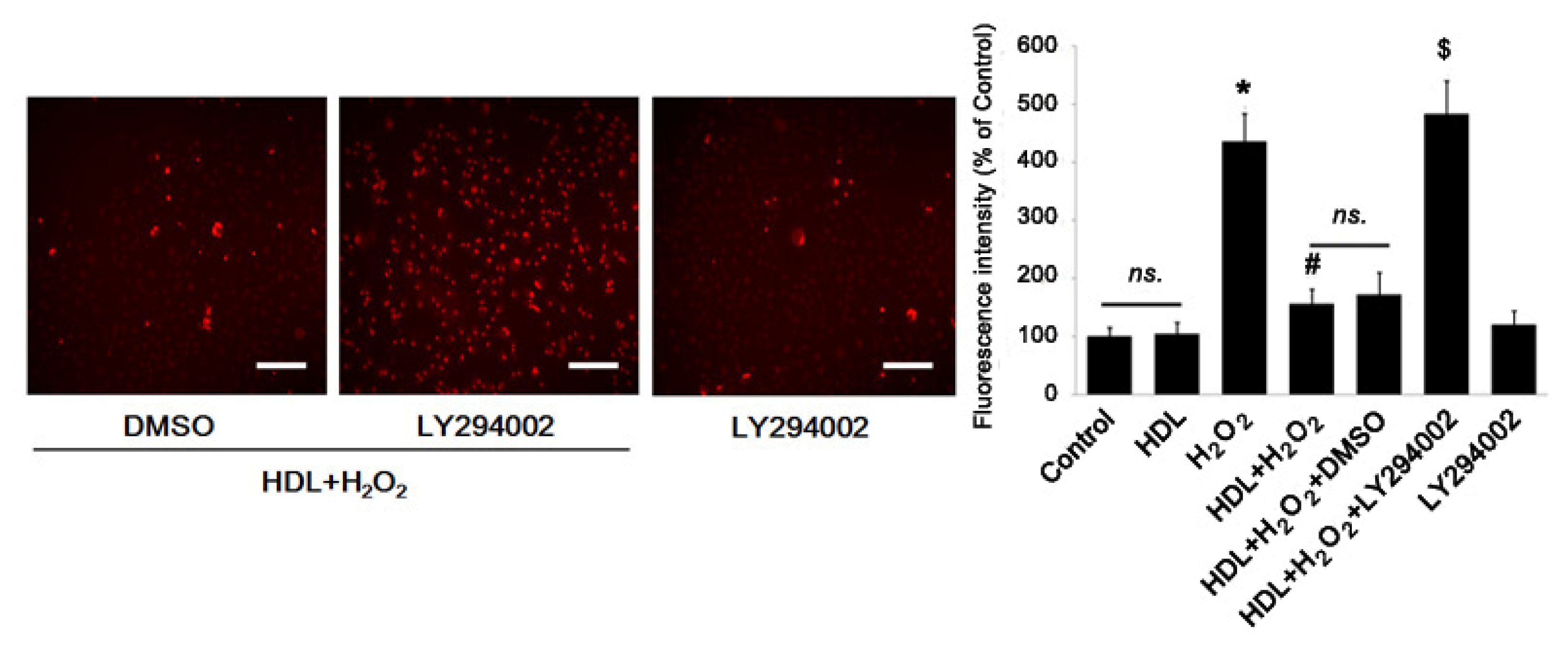
2.3. Phosphorylation of Akt Induced by HDL Inhibits ROS Generation
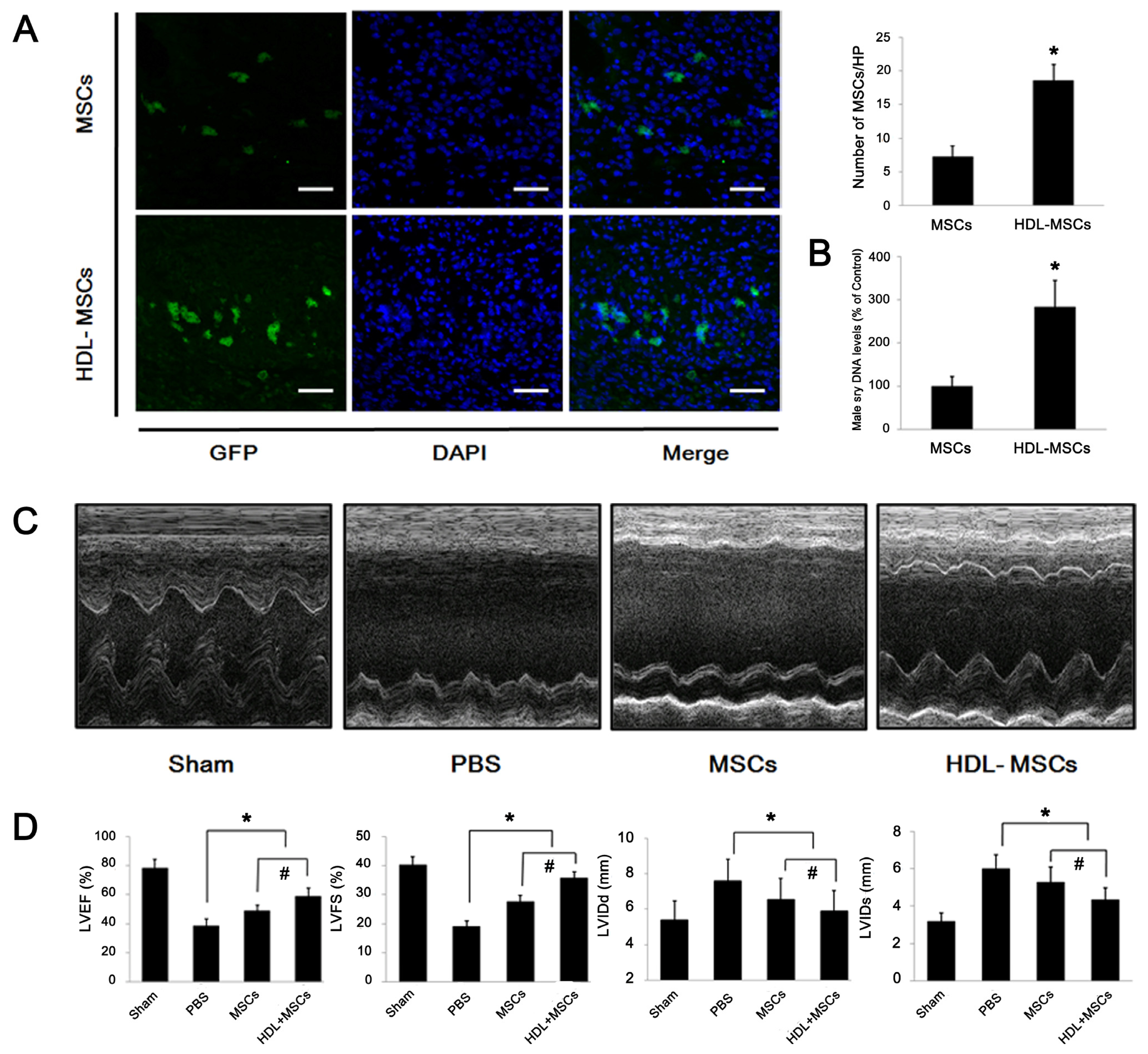
2.4. HDL Pre-Incubation Promotes MSC Survival in the Infarcted Heart
2.5. MSCs Preconditioned with HDL Reduce Cardiac Remodeling and Preserve Myocardial Function
3. Experimental Section
3.1. Isolation and Culture of MSCs
3.3. In vitro Exposure to H2O2 and HDL
3.4. MSC Viability Assay
3.5. TUNEL (Terminal Dexynucleotidyl Transferase-Mediated dUTP Nick end Labeling) Assay
3.6. Caspase-3 Activity Assay
3.7. Senescence-Associated β-Galactosidase Staining
3.8. Western Blot Analysis
3.9. Measurement of ROS
3.10. Myocardial Infarction and MSCs Transplantation
3.11. Tracking of the GFP+ MSCs Injected in MI Hearts
3.12. Real-Time Polymerase Chain Reaction
3.13. Echocardiography
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
ijms-13-17104-s001.pdfAcknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Huang, J.; Zhang, Z.; Guo, J.; Ni, A.; Deb, A.; Zhang, L.; Mirotsou, M.; Pratt, R.E.; Dzau, V.J. Genetic Modification of Mesenchymal Stem Cells Overexpressing CCR1 Increases Cell Viability, Migration, Engraftment, and Capillary Density in the Injured Myocardium. Circ. Res 2010, 106, 1753–1762. [Google Scholar]
- Wen, Z.Z.; Zheng, S.X.; Zhou, C.Q.; Yuan, W.L.; Wang, J.F.; Wang, T. Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: MicroRNAs as novel regulators. J. Cell. Mol. Med 2012, 16, 657–671. [Google Scholar]
- Luo, Y.; Wang, Y.; Poynter, J.A.; Manukyan, M.C.; Herrmann, J.L.; Abarbanell, A.M.; Weil, B.R.; Meldrum, D.R. Pretreating mesenchymal stem cells with interleukin-1 beta and transforming growth factor-beta synergistically increases vascular endothelial growth factor production and improves mesenchymal stem cell-mediated myocardial protection after acute ischemia. Surgery 2012, 151, 353–363. [Google Scholar]
- Wei, H.; Li, Z.; Hu, S.; Chen, X.; Cong, X. Apoptosis of Mesenchymal Stem Cells Induced by Hydrogen Peroxide Concerns Both Endoplasmic Reticulum Stress and Mitochondrial Death Pathway Through Regulation of Calspases, p38 and JNK. J. Cell. Biochem 2010, 111, 967–978. [Google Scholar]
- Wang, X.; Zhao, T.; Huang, W.; Wang, T.; Qian, J.; Xu, M.; Kranias, E.G.; Wang, Y.; Fan, G.-C. Hsp20-Engineered Mesenchymal Stem Cells Are Resistant to Oxidative Stress via Enhanced Activation of Akt and Increased Secretion of Growth Factors. Stem Cells 2009, 27, 3021–3031. [Google Scholar]
- Zanichelli, F.; Capasso, S.; di Bernardo, G.; Cipollaro, M.; Pagnotta, E.; Carteni, M.; Casale, F.; Iori, R.; Giordano, A.; Galderisi, U. Low concentrations of isothiocyanates protect mesenchymal stem cells from oxidative injuries, while high concentrations exacerbate DNA damage. Apoptosis 2012, 17, 964–974. [Google Scholar]
- Duffy, D.; Holmes, D.N.; Roe, M.T.; Peterson, E.D. The impact of high-density lipoprotein cholesterol levels on long-term outcomes after non-ST-elevation myocardial infarction. Am. Heart J 2012, 163, 705–713. [Google Scholar]
- Navab, M.; Reddy, S.T.; van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol 2011, 8, 222–232. [Google Scholar]
- Lin, L.; Gong, H.; Ge, J.; Jiang, G.; Zhou, N.; Li, L.; Ye, Y.; Zhang, G.; Ge, J.; Zou, Y. High density lipoprotein downregulates angiotensin II type 1 receptor and inhibits angiotensin II-induced cardiac hypertrophy. Biochem. Biophys. Res. Commun 2011, 404, 28–33. [Google Scholar]
- Nicholls, S.J.; Dusting, G.J.; Cutri, B.; Bao, S.; Drummond, G.R.; Rye, K.A.; Barter, P.J. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 2005, 111, 1543–1550. [Google Scholar]
- De Souza, J.A.; Vindis, C.; Negre-Salvayre, A.; Rye, K.-A.; Couturier, M.; Therond, P.; Chantepie, S.; Salvayre, R.; Chapman, M.J.; Kontush, A. Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: pivotal role of apolipoprotein A-I. J. Cell. Mol. Med 2010, 14, 608–620. [Google Scholar]
- Sumi, M.; Sata, M.; Miura, S.-I.; Rye, K.-A.; Toya, N.; Kanaoka, Y.; Yanaga, K.; Ohki, T.; Saku, K.; Nagai, R. Reconstituted high-density lipoprotein stimulates differentiation of endothelial progenitor cells and enhances ischemia-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol 2007, 27, 813–818. [Google Scholar]
- Ng, K.-M.; Lee, Y.-K.; Lai, W.-H.; Chan, Y.-C.; Fung, M.-L.; Tse, H.-F.; Siu, C.-W. Exogenous Expression of Human apoA-I Enhances Cardiac Differentiation of Pluripotent Stem Cells. PLoS One 2011, 6, e19787. [Google Scholar]
- Yvan-Charvet, L.; Pagler, T.; Gautier, E.L.; Avagyan, S.; Siry, R.L.; Han, S.; Welch, C.L.; Wang, N.; Randolph, G.J.; Snoeck, H.W.; et al. ATP-Binding Cassette Transporters and HDL Suppress Hematopoietic Stem Cell Proliferation. Science 2010, 328, 1689–1693. [Google Scholar]
- Wang, B.; Shravah, J.; Luo, H.; Raedschelders, K.; Chen, D.D.Y.; Ansley, D.M. Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem. Biophys. Res. Commun 2009, 389, 105–111. [Google Scholar]
- Brandl, A.; Meyer, M.; Bechmann, V.; Nerlich, M.; Angele, P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp. Cell. Res 2011, 317, 1541–1547. [Google Scholar]
- Di Bernardo, G.; Alessio, N.; Dell’Aversana, C.; Casale, F.; Teti, D.; Cipollaro, M.; Altucci, L.; Galderisi, U. Impact of Histone Deacetylase Inhibitors SAHA and MS-275 on DNA Repair Pathways in Human Mesenchymal Stem Cells. J. Cell. Physiol 2010, 225, 537–544. [Google Scholar]
- Zhu, Z.; Sun, H.; Ma, G.; Wang, Z.; Li, E.; Liu, Y.; Liu, Y. Bufalin Induces Lung Cancer Cell Apoptosis via the Inhibition of PI3K/Akt Pathway. Int. J. Mol. Sci 2012, 13, 2025–2035. [Google Scholar]
- Li, S.; Bian, H.; Liu, Z.; Wang, Y.; Dai, J.; He, W.; Liao, X.; Liu, R.; Luo, J. Chlorogenic acid protects MSCs against oxidative stress by altering FOXO family genes and activating intrinsic pathway. Eur. J. Pharmacol 2012, 674, 65–72. [Google Scholar]
- Tam, D.N.N.; Son, Y.O.; Lim, S.S.; Shi, X.L.; Kim, J.G.; Heo, J.S.; Choe, Y.; Jeon, Y.M.; Lee, J.C. Sodium fluoride induces apoptosis in mouse embryonic stem cells through ROS-dependent and caspase- and JNK-mediated pathways. Toxicol. Appl. Pharmacol 2012, 259, 329–337. [Google Scholar]
- Kiya, Y.; Miura, S.-I.; Imaizumi, S.; Uehara, Y.; Matsuo, Y.; Abe, S.; Jimi, S.; Urata, H.; Rye, K.-A.; Saku, K. Reconstituted high-density lipoprotein attenuates postinfarction left ventricular remodeling in rats. Atherosclerosis 2009, 203, 137–144. [Google Scholar]
- Xie, X.X.; Sun, A.J.; Zhu, W.Q.; Huang, Z.Y.; Hu, X.Y.; Jia, J.G.; Zou, Y.Z.; Ge, J.B. Transplantation of Mesenchymal Stem Cells Preconditioned with Hydrogen Sulfide Enhances Repair of Myocardial Infarction in Rats. Tohoku J. Exp. Med 2012, 226, 29–36. [Google Scholar]
- Mangi, A.A.; Noiseux, N.; Kong, D.L.; He, H.M.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med 2003, 9, 1195–1201. [Google Scholar]
- Suzuki, Y.; Kim, H.W.; Ashraf, M.; Haider, H.K. Diazoxide potentiates mesenchymal stem cell survival via NF-κ B-dependent miR-146a expression by targeting Fas. Am. J. Physiol. Heart Circul. Physiol 2010, 299, H1077–H1082. [Google Scholar]
- Rossoni, G.; Gomaraschi, M.; Berti, F.; Sirtori, C.R.; Franceschini, G.; Calabresi, L. Synthetic high-density lipoproteins exert cardioprotective effects in myocardial ischemia/reperfusion injury. J. Pharmacol. Exp. Ther 2004, 308, 79–84. [Google Scholar]
- Strandberg, T.E.; Saijonmaa, O.; Fyhrquist, F.; Tilvis, R.S.; Strandberg, A.Y.; Miettinen, T.A.; Pitkala, K.H.; Salomaa, V. Telomere length in old age and cholesterol across the life course. J. Am.Geriatr. Soc 2011, 59, 1979–1981. [Google Scholar]
- Barzilai, N.; Atzmon, G.; Schechter, C.; Schaefer, E.J.; Cupples, A.L.; Lipton, R.; Cheng, S.; Shuldiner, A.R. Unique lipoprotein phenotype and genotype associated with exceptional longevity. J. Am. Med. Assoc 2003, 290, 2030–2040. [Google Scholar]
- Tian, L.; Fu, M. The relationship between high density lipoprotein subclass profile and apolipoprotein concentrations. J. Endocrinol. Invest 2011, 34, 461–472. [Google Scholar]
- Terasaka, N.; Wang, N.; Yvan-Charvet, L.; Tall, A.R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA 2007, 104, 15093–15098. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, J.; Qian, J.; Xie, X.; Lin, L.; Zou, Y.; Fu, M.; Huang, Z.; Zhang, G.; Su, Y.; Ge, J. High Density Lipoprotein Protects Mesenchymal Stem Cells from Oxidative Stress-Induced Apoptosis via Activation of the PI3K/Akt Pathway and Suppression of Reactive Oxygen Species. Int. J. Mol. Sci. 2012, 13, 17104-17120. https://doi.org/10.3390/ijms131217104
Xu J, Qian J, Xie X, Lin L, Zou Y, Fu M, Huang Z, Zhang G, Su Y, Ge J. High Density Lipoprotein Protects Mesenchymal Stem Cells from Oxidative Stress-Induced Apoptosis via Activation of the PI3K/Akt Pathway and Suppression of Reactive Oxygen Species. International Journal of Molecular Sciences. 2012; 13(12):17104-17120. https://doi.org/10.3390/ijms131217104
Chicago/Turabian StyleXu, Jianfeng, Juying Qian, Xinxing Xie, Li Lin, Yunzeng Zou, Mingqiang Fu, Zheyong Huang, Guoping Zhang, Yangang Su, and Junbo Ge. 2012. "High Density Lipoprotein Protects Mesenchymal Stem Cells from Oxidative Stress-Induced Apoptosis via Activation of the PI3K/Akt Pathway and Suppression of Reactive Oxygen Species" International Journal of Molecular Sciences 13, no. 12: 17104-17120. https://doi.org/10.3390/ijms131217104



