Pharmacological Mechanisms Underlying Gastroprotective Activities of the Fractions Obtained from Polygonum minus in Sprague Dawley Rats
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effect of Polygonum minus Fractions on Gastric Lesions in the Ethanol Induction Model
2.2. Effect of Polygonum minus Fractions on the Mucus Wall Barrier
2.3. Effect of Polygonum minus Fractions on PGE2 Synthesis
2.4. Effect of the Polygonum minus Fractions on the Hexosamine Level
2.5. Effect of Polygonum minus Fractions on Superoxide Dismutase (SOD) Activity
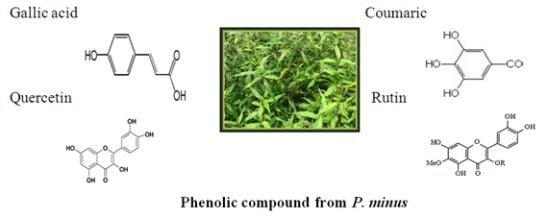
2.6. Phytochemical Analysis
3. Experimental
3.1. Plant Extraction and Fractionation
3.2. Experimental Protocol for Anti-Ulcer Evaluation
3.3. Evaluation of Gross and Histological Changes
3.4. Determination of Gastric Wall Mucus
3.5. Preparation of Stomach Tissue Homogenate
3.5.1. Determination of Prostaglandin Synthesis
3.5.2. Determination of Total Hexosamine
3.5.3. Determination of Superoxide Dismutase (SOD)
3.6. Phytochemical Analysis
3.6.1. HPLC
3.6.2. UPLC-ESI-MS/MS
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
References
- Klein, L.C.; Gandolfi, R.B.; Santin, J.R.; Lemos, M.; Filho, V.C.; de Andrade, S.F. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Polygalaceae). Naunyn-Schmiedeberg’s Arch. Pharmacol 2010, 381, 121–126. [Google Scholar]
- Bandyopadhyay, D.; Biswas, K.; Reiter, R.J.; Banerjee, R.K. Gastric toxicity and mucosal ulceration induced by oxygen-derived reactive species: protection by melatonin. Curr. Mol. Med 2001, 1, 501–513. [Google Scholar]
- Kaunitz, J.D.; Akiba, Y. Gastroduodenal mucosal defense: role of endogenous mediators. Curr. Opin. Gastroen 2004, 20, 526–532. [Google Scholar]
- Tarnawski, A.S. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci 2005, 50, 24–33. [Google Scholar]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture: Kuala Lumpur, Malaysia, 1966; Volume 2, 1823.
- Faujan, N.H.; Abdullah, N.; Abdullah Sani, A.; Babji, A.M. Antioxidative activities of water extracts of some Malaysian herbs. ASEAN Food J 2007, 14, 61–68. [Google Scholar]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Najim, N.; Zain, M.M.; Hamdan, S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules 2011, 16, 3433–3443. [Google Scholar]
- Gor, M.C.; Ismail, I.; Mustapha, W.A.W.; Zainal, Z.; Noor, N.M.; Othman, R.; Hussein, Z.A.M. Identification of cDNAs for jasmonic acid-responsive genes in Polygonum minus roots by suppression subtractive hybridization. Acta Physiol. Plant 2011, 33, 283–294. [Google Scholar]
- Wasman, S.Q.; Mahmood, A.A.; Salehhuddin, H.; Zahra, A.A.; Salmah, I. Cytoprotective activities of Polygonum minus aqueous leaf extract on ethanol-induced gastric ulcer in rats. J. Med. Plants Res 2010, 4, 2658–2665. [Google Scholar]
- Uyub, A.M.; Nwachukwu, I.N.; Azlan, A.A.; Fariza, S.S. In-vitro antibacterial activity and cytotoxicity of selected medicinal plant extracts from Penang Island Malaysia on metronidazole-resistant-Helicobacter pylori and some pathogenic bacteria. Ethnobot. Res. Appl 2010, 8, 95–106. [Google Scholar]
- Ali, A.M.; Mackeen, M.M.; El-Sharkawy, S.H.; Hamid, J.A.; Ismail, N.H.; Ahmad, F.; Lajis, M.N. Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika J. Trop. Agric. Sci 1996, 19, 129–136. [Google Scholar]
- Kinoshita, M.; Noto, T.; Tamaki, H. Effect of a combination of ecabet sodium and cimetidine on experimentally induced gastric lesions and gastric mucosal resistance to ulcerogenic agents in rats. Biol. Pharm. Bull 1995, 18, 223–226. [Google Scholar]
- Murata, H.; Kawano, S.; Tsuji, S.; Kamada, T.; Matsuzawa, Y.; Katsu, K.; Inoue, K.; Kobayashi, K.; Mitsufuji, S.; Bamba, T; et al. Combination therapy of ecabet sodium and cimetidine compared with cimetidine alone for gastric ulcer: Prospective randomized multicenter study. J. Gastroen. Hepatol. 2003, 18, 1029–1033. [Google Scholar]
- García-Barrantes, P.M.; Badilla, B. Anti-ulcerogenic properties of Quassia amara L.(Simaroubaceae) standardized extracts in rodent models. J. Ethnopharmacol 2011, 134, 904–910. [Google Scholar]
- Alqasoumi, S.; Al-Sohaibani, M.; Al-Howiriny, T.; Al-Yahya, M.; Rafatullah, S. Rocket “Eruca sativa”: A salad herb with potential gastric anti-ulcer activity. World J. Gastroenterol 2009, 15, 1958–1965. [Google Scholar]
- German, M.; D’Angelo, V.; Biasini, T.; Miano, T.; Braca, A.; de Leo, M.; de Pasquale, R.; Sanogo, R. Anti-ulcer, anti-inflammatory and antioxidant activities of the n-butanol fraction from Pteleopsis suberosa stem bark. J. Ethnopharmacol 2008, 115, 271–275. [Google Scholar]
- Gorinstein, S.; Vargas, O.J.M.; Jaramillo, N.O.; Salas, I.A.; Ayala, A.L.M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur. Food Res. Technol 2007, 225, 321–328. [Google Scholar]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar]
- Miller, J.P.; Faragher, E.B. Relapse of duodenal ulcer: Does it matter which drug is used in initial treatment? Br. Med. J 1986, 293, 1117–1118. [Google Scholar]
- Ariyphisi, I.; Toshiharu, A.; Sugimura, F.; Abe, M.; Matsuo, Y.; Honda, T. Recurrence during maintenance therapy with histamine H2 receptors antagonist in cases of gastric ulcers. Nikon Univ. J. Med 1983, 28, 69–74. [Google Scholar]
- Reyes-Trejo, B.; Sánchez-Mendoza, M.E.; Becerra-García, A.A.; Cedillo-Portugal, E.; Castillo-Henkel, C.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer diterpenoid from Croton reflexifolius: Role of nitric oxide, prostaglandins and sulfhydryls. J. Pharm. Pharmacol 2008, 60, 931–936. [Google Scholar]
- Nanjundaiah, S.M.; Annaiah, H.N.; Dharmesh, S.M. Gastroprotective effect of ginger rhizome (Zingiber officinale) extract: Role of gallic acid and cinnamic acid in H+, K+-ATPase/H. pylori inhibition and anti-oxidative mechanism. Evid. Based Complement. Alternat. Med 2011. [Google Scholar] [CrossRef]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Ochi, M.; Yoshikawa, M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. Eur. J. Pharmacol 2003, 471, 59–67. [Google Scholar]
- de Sousa Falcão, H.; Leite, J.A.; Barbosa-Filho, J.M.; de Athayde-Filho, P.F.; de Oliveira Chaves, M.C.; Moura, M.D.; Ferreira, A.L.; de Almeida, A.B.A.; Souza-Brito, A.R.M.; de Fátima Formiga Melo Diniz, M.; et al. Gastric and duodenal antiulcer activity of alkaloids: A review. Molecules 2008, 13, 3198–3223. [Google Scholar]
- Xia, J. Medicinal herbs used in pairs for treatment of 98 cases of chronic gastritis. J. Tradit. Chin. Med 2004, 24, 208–209. [Google Scholar]
- Chakŭrski, I.; Matev, M.; Stefanov, G.; Koĭchev, A.; Angelova, I. Treanntment of duodenal ulcers and gastroduodenitis with a herbal combination of Symphitum officinalis and Calendula officinalis with and without antacids. Vutr. Boles 1981, 20, 44–47. [Google Scholar]
- Chernomorets, N.N.; Seleznev, A.V.; Revutskiĭ, B.I.; Alifanova, R.E.; Kravchenko, Z.V.; Cherkasskaia, E.P. The differentiated phytotherapy of patients with duodenal peptic ulcer. Lik. Sprava 1992, 2, 112–115. [Google Scholar]
- Hiruma-Lima, C.A.; Calvo, T.R.; Rodrigues, C.M.; Andrade, F.D.P.; Vilegas, W.; Brito, A.R.M.S. Antiulcerogenic activity of Alchornea castaneaefolia: Effects on somatostatin, gastrin and prostaglandin. J. Ethnopharmacol 2006, 104, 215–224. [Google Scholar]
- Banerjee, D.; Bauri, A.K.; Guha, R.K.; Bandyopadhyay, S.K.; Chattopadhyay, S. Healing properties of malabaricone B and malabaricone C, against indomethacin-induced gastric ulceration and mechanism of action. Eur. J. Pharmacol 2008, 578, 300–312. [Google Scholar]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; Sánchez-Gómez, P.; Rodríguez-Silverio, J.; Castillo-Henkel, C.; Cervantes-Cuevas, H.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschenbornianum: Role of nitric oxide, prostaglandins and sulfydryls. Fitoterapia 2010, 81, 66–71. [Google Scholar]
- Bhattacharya, S.; Chaudhuri, S.R.; Chattopadhyay, S.; Bandyopadhyay, S.K. Healing properties of some Indian medicinal plants against indomethacin-induced gastric ulceration of rats. J. Clin. Biochem. Nutr 2007, 41, 106–114. [Google Scholar]
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol 2009, 8, 484–489. [Google Scholar]
- Garg, G.P.; Nigam, S.K.; Ogle, C.W. The gastric antiulcer effects of the leaves of the neem tree. Planta Med 1993, 59, 215–217. [Google Scholar]
- Njar, V.C.O.; Adesanwo, J.K.; Raji, Y. Methyl angolenate: The antiulcer agent from the stem bark of Entandrophragma angolense. Planta Med 1995, 61, 91–92. [Google Scholar]
- Mahmood, A.; Mariod, A.A.; Al-Bayaty, F.; Abdel-Wahab, S.I. Anti-ulcerogenic activity of Gynura procumbens leaf extract against experimentally-induced gastric lesions in rats. J. Med. Plant. Res 2010, 4, 685–691. [Google Scholar]
- Corne, S.J.; Morrissey, S.M.; Woods, R.J. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J. Physiol 1974, 242, 116–117. [Google Scholar]
- Al-Qarawi, A.A.; Abdel-Rahman, H.; Ali, B.H.; Mousa, H.M.; El-Mougy, S.A. The ameliorative effect of dates (Phoenix dactylifera L.) on ethanol-induced gastric ulcer in rats. J. Ethnopharmacol 2005, 98, 313–317. [Google Scholar]
- Jang, J.H.; Hia, H.C.; Ike, M.; Inoue, C.; Fujita, M.; Yoshida, T. Acid hydrolysis and quantitative determination of total hexosamines of an exopolysaccharide produced by Citrobacter sp. Biotechnol. Lett 2005, 27, 13–18. [Google Scholar]
- Wang, H.; Helliwell, K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res. Int 2001, 34, 223–227. [Google Scholar]
- Liza, M.S.; Rahman, R.A.; Mandana, B.; Jinap, S.; Rahmat, A.; Zaidul, I.S.M.; Hamid, A. Supercritical carbon dioxide extraction of bioactive flavonoid from Strobilanthes crispus (Pecah Kaca). Food Bioprod. Process 2010, 88, 319–326. [Google Scholar]
- Chua, L.S.; Latiff, N.A.; Lee, S.Y.; Lee, C.T.; Sarmidi, M.R.; Aziz, R.A. Flavonoids and phenolic acids from Labisia pumila (Kacip Fatimah). Food Chem 2011, 127, 1186–1192. [Google Scholar]
- Statistical Package Social Science, version 17.0; SAS Institute Inc: Cary, NC, USA, 2009.
- GraphPad PRISM, version 5.0; Graph Pad Software, Inc.: San Diego, CA, USA, 2007.










© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Sirat, H.M.; Hamdan, S. Pharmacological Mechanisms Underlying Gastroprotective Activities of the Fractions Obtained from Polygonum minus in Sprague Dawley Rats. Int. J. Mol. Sci. 2012, 13, 1481-1496. https://doi.org/10.3390/ijms13021481
Qader SW, Abdulla MA, Chua LS, Sirat HM, Hamdan S. Pharmacological Mechanisms Underlying Gastroprotective Activities of the Fractions Obtained from Polygonum minus in Sprague Dawley Rats. International Journal of Molecular Sciences. 2012; 13(2):1481-1496. https://doi.org/10.3390/ijms13021481
Chicago/Turabian StyleQader, Suhailah Wasman, Mahmood Ameen Abdulla, Lee Suan Chua, Hasnah Mohd Sirat, and Salehhuddin Hamdan. 2012. "Pharmacological Mechanisms Underlying Gastroprotective Activities of the Fractions Obtained from Polygonum minus in Sprague Dawley Rats" International Journal of Molecular Sciences 13, no. 2: 1481-1496. https://doi.org/10.3390/ijms13021481