Preparation of TiO2 Nanocrystallite Powders Coated with 9 mol% ZnO for Cosmetic Applications in Sunscreens
Abstract
:1. Introduction
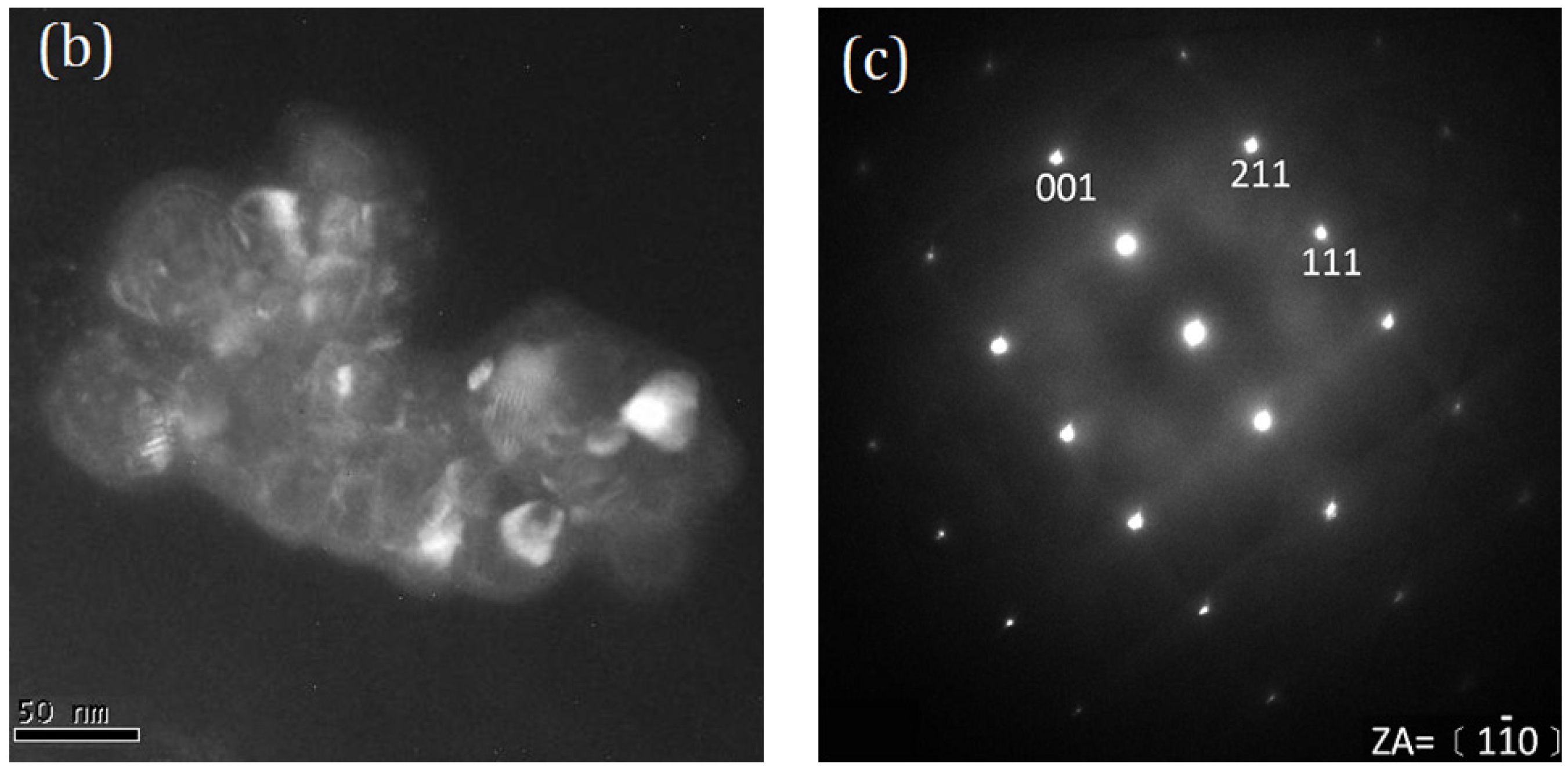
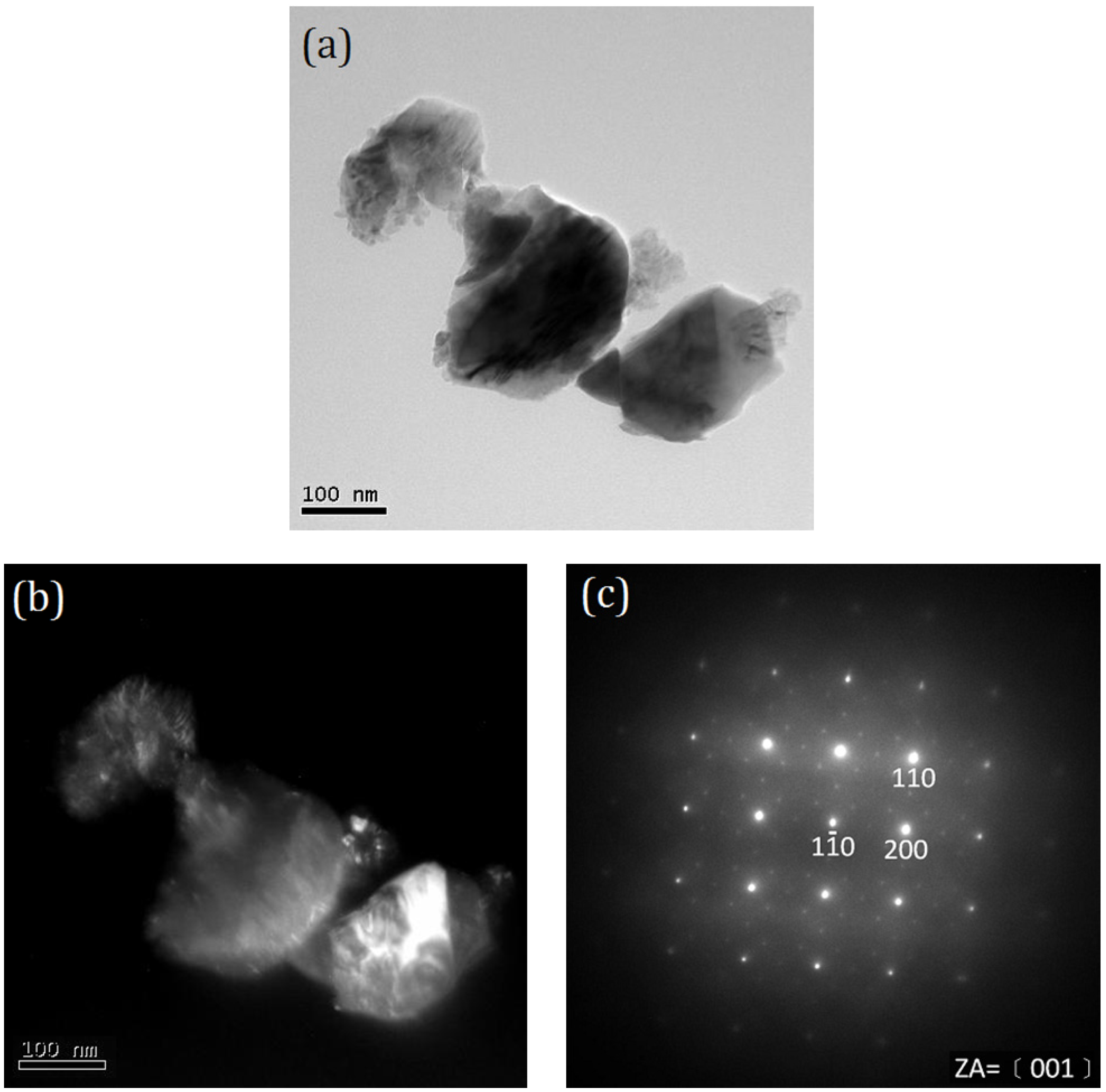
2. Results and Discussion
3. Experimental Procedure
3.1. Sample Preparation
3.2. Sample Characterization
4. Conclusions
- The rutile TiO2 began to form at 523 K of T-0Z freeze dried precursor powders, and was the dominant phase when calcined at 973 K for 2 h.
- The anatase and rutile TiO2 phases coexist in T-9Z powders when the calcination temperature is below 973 K. When calcined at 1273 K for 2 h, the anatase phase disappears. In addition, the Zn2Ti3O8 first forms when T-9Z freeze dried precursor powders are calcined at 973 K for 2 h. When the T-9Z precursor powders are calcined at 1273 K for 2 h, the Zn2TiO4 forms and the Zn2Ti3O8 disappears.
- The average crystallite sizes of anatase and rutile TiO2 increase with increasing the calcination temperature, but all average crystallite sizes of anatase and rutile TiO2 are smaller than 100 nm for T-0Z and T-9Z freeze dried precursor powders as calcined between 523 and 1275 K for 2 h. In addition, the crystallite size of rutile TiO2 in T-0Z is smaller than that in T-9Z when the freeze dried precursor powders are calcined at 1273 K for 2 h.
- The absorption of T-9Z powders in the UV range has a red-shift effect as the calcination temperature increases. This result shows that TiO2 nanocrystallite powders added with 9 mol% ZnO and calcined at 1273 K for 2 h can be used as an UV-A attenuating agent for cosmetic applications in sunscreens.
Acknowledgments
References
- Al-Hilli, S.M.; Willander, M. Optical properties of zinc oxide nano-particle embedded in dielectric medium for UV region: Numerical simulation. J. Nanoparticle Res 2006, 8, 79–97. [Google Scholar]
- Nohynek, G.J.; Lademann, J.; Ribaud, C.; Roberts, M.S. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol 2007, 37, 251–277. [Google Scholar]
- Lee, W.; Shen, H.S.; Dwight, K.; Wold, A. Effect of silver on the photocatalytic activity of TiO2. J. Solid State Chem 1998, 106, 288–294. [Google Scholar]
- Fairhurst, D.; Mitchnick, M.A. Sunscreen-Development, Evaluation and Regulatory Aspects, 2nd ed; Lowe, N.J., Shaath, N.A., Pathak, M.A., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 3–33. [Google Scholar]
- Zhong, C.J.; Maye, M.M. Core-shell assembled nanoparticles as catalysis. Adv. Mater 2001, 13, 1507–1511. [Google Scholar]
- Jaroenworaluck, A.; Sunsaneeyamethe, W.; Kosachan, N.; Stevens, R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf. Interface Anal 2006, 38, 473–477. [Google Scholar]
- Liao, M.H.; Hsu, C.H.; Chen, D.H. Preparation and properties of amorphous titania-coated zinc oxide nanoparticles. J. Solid State Chem 2006, 179, 2020–2026. [Google Scholar]
- Zhang, H.; Banfield, J.F. Phase transformation of nanocrystalline anatase-to-rutile via combined interface and surface nucleation. J. Mater. Res 2000, 15, 437–448. [Google Scholar]
- Li, G.; Li, L.; Boerio-Goates, J.; Woodfield, B.F. High purity anatase nanocrystals: Near room temperature synthesis, grain growth kinetics, and surface hydration chemistry. J. Am. Chem. Soc 2005, 127, 8659–8666. [Google Scholar]
- Dulin, P.H.; Rase, D.E. Phase equilibria in the system ZnO-TiO2. J. Am. Ceram. Soc 1960, 43, 125–131. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed; Addison-Wesley Publishing Company: Reading, MA, USA, 1978; p. 87. [Google Scholar]
- Wilke, K.; Breuer, H.D. The influence of transition metal doping on the physical and photocatalytic properties of titania. J. Photochem. Photobiol. A 1999, 121, 49–53. [Google Scholar]
- Zhou, Y.S.; Fan, X.H. Preparation and properties of the nanometer composite oxide TiO2-SiO2. Chem. J. Chin. Univ 2003, 7, 1266–1270. [Google Scholar]
- Jing, L.; Sun, X.; Cai, W.; Li, X.; Fu, H.; Hou, H.; Fan, N. Photoluminescence of Ce doped TiO2 nanoparticles and their photocatalytic activity. Acta Chim. Sin 2003, 61, 1241–1245. [Google Scholar]
- Yan, Q.Z.; Su, X.T.; Huang, Z.Y.; Ge, C.C. Sol-gel auto-igniting synthesis and structural property of cerium-doped titanium dioxide nanosized powders. J. Eur. Ceram. Soc 2006, 2b, 915–921. [Google Scholar]
- Hirano, M.; Joji, T.; Inagaki, M. Direct formation of iron (III)-doped titanium oxide (anatase) by thermal hydrolysis and its structure property. J. Am. Ceram. Soc 2004, 87, 35–41. [Google Scholar]
- Gesenhues, U.; Rentschler, T. Crystal growth and defect structure of Al3+-doped rutile. J. Solid. State Chem 1999, 143, 210–218. [Google Scholar]
- Inagaki, M.; Nakazawa, Y.; Hirano, M.; Kobayashi, Y.; Toyoda, M. Preparation of stable anatase-type TiO2 and its photocatalytic performance. Int. J. Inorg. Mater 2001, 3, 809–811. [Google Scholar]
- Chang, Y.S.; Chang, Y.H.; Chen, I.G.; Chen, G.J.; Chai, Y.L. Synthesis and characterization of zinc titanate nano-crystal powders by sol-gel technique. J. Cryst. Growth 2006, 243, 319–326. [Google Scholar]
- Wang, C.L.; Hwang, W.S.; Chang, K.M.; Ko, H.K.; Hsi, C.S.; Huang, H.H.; Wang, M.C. Formation and morphology of Zn2Ti3O8 powders using hydrothermal process without dispersant agent or mineralizer. Int. J. Mol. Sci 2011, 12, 935–945. [Google Scholar]
- Maria, A.J.; Yeung, K.L.; Lee, C.Y.; Yue, P.L.; Chan, C.K. Size effect in gas-phase photo-oxidation of trichloroethylene using nanometer-sized TiO2 catalysts. J. Catal 2000, 192, 185–196. [Google Scholar]
- Herzog, B.; Quass, K.; Schmidt, E.; Mller, S.; Luther, H. Physical properties of organic particulate UV absorbers used in sunscreens II. UV-attenuating efficiency as function of particle size. J. Colloid Interface Sci 2004, 276, 354–363. [Google Scholar]
- Robb, J.L.; Simpson, L.H.; Tunstall, D.F. Scattering & absorption of UV radiation by sunscreens containing fine particle & pigmentary titanium dioxide. Drug Cosmet. Ind 1994, 154, 32–39. [Google Scholar]
- Popov, A.P.; Priezzhev, A.V.; Lademann, J.; Myllyla, R. TiO2 nanoparticles as an effective UV-B radiation skin-protective compound in sunscreens. J. Phys. D 2005, 38, 2564–2570. [Google Scholar]
- Kuo, C.L.; Wang, C.L.; Ko, H.H.; Hwang, W.S.; Chang, K.M.; Li, W.L.; Huang, H.H.; Chang, Y.H.; Wang, M.C. Synthesis of zinc oxide nanocrystalline powders for cosmetic applications. Ceram. Int 2010, 36, 693–698. [Google Scholar]





| Calcination Temperature (K) | Crystallite Size T-0Z (nm) | Crystallite Size T-9Z (nm) | ||
|---|---|---|---|---|
| Anatase | Rutile | Anatase | Rutile | |
| 523 | 6.5 ± 0.2 | - | 5.0 ± 0.2 | 5.7 ± 0.2 |
| 673 | 9.8 ± 0.3 | 13.2 ± 0.2 | 8.6 ± 0.2 | 10.0 ± 0.2 |
| 773 | 15.5 ± 0.3 | 21.0 ± 0.4 | 12.9 ± 0.2 | 15.7 ± 0.2 |
| 873 | 16.5 ± 0.3 | 28.9 ± 0.4 | 31.4 ± 0.4 | 34.0 ± 0.4 |
| 973 | 20.4 ± 0.4 | 37.4 ± 0.5 | - | 48.6 ± 0.4 |
| 1273 | - | 57.7 ± 0.6 | - | 78.8 ± 0.6 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ko, H.-H.; Chen, H.-T.; Yen, F.-L.; Lu, W.-C.; Kuo, C.-W.; Wang, M.-C. Preparation of TiO2 Nanocrystallite Powders Coated with 9 mol% ZnO for Cosmetic Applications in Sunscreens. Int. J. Mol. Sci. 2012, 13, 1658-1669. https://doi.org/10.3390/ijms13021658
Ko H-H, Chen H-T, Yen F-L, Lu W-C, Kuo C-W, Wang M-C. Preparation of TiO2 Nanocrystallite Powders Coated with 9 mol% ZnO for Cosmetic Applications in Sunscreens. International Journal of Molecular Sciences. 2012; 13(2):1658-1669. https://doi.org/10.3390/ijms13021658
Chicago/Turabian StyleKo, Horng-Huey, Hui-Ting Chen, Feng-Ling Yen, Wan-Chen Lu, Chih-Wei Kuo, and Moo-Chin Wang. 2012. "Preparation of TiO2 Nanocrystallite Powders Coated with 9 mol% ZnO for Cosmetic Applications in Sunscreens" International Journal of Molecular Sciences 13, no. 2: 1658-1669. https://doi.org/10.3390/ijms13021658




