Isolation and Characterization of Activators of ERK/MAPK from Citrus Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts from Citrus Species
2.2. Preparation of Extracts of Citrus Grandis (Kawachi bankan)
2.3. Analysis of n-hexane Extracts of Citrus Grandis
2.4. Preparation of HMF from Orange Oil
2.5. Cell Cultures and Reagents
2.6. Immunoblot Analysis
2.7. MTT Assay
2.8. Animals and Drugs
2.9. Morris-Type Water Maze (MWM) Test
3. Results and Discussion
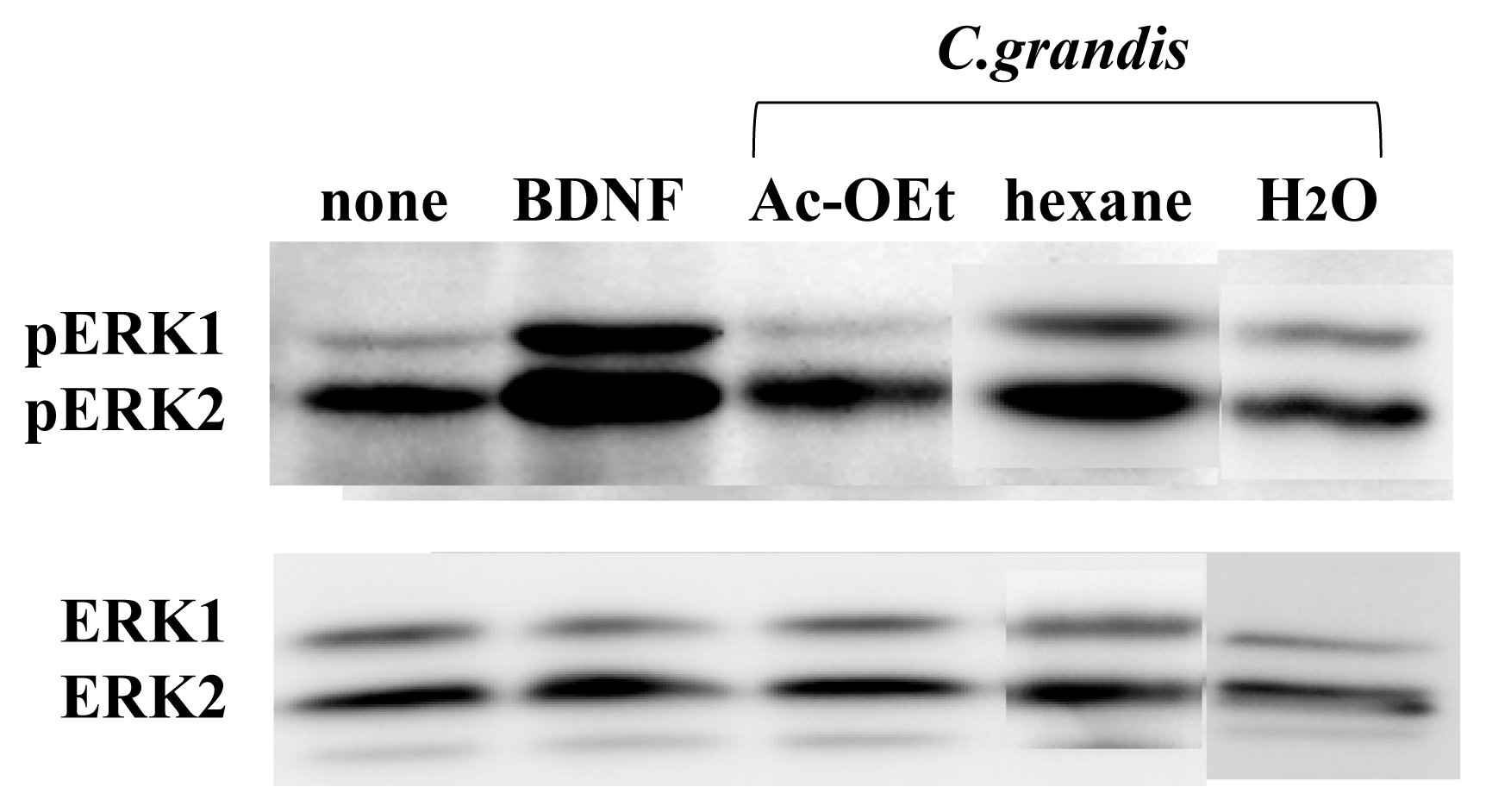
3.1. ERK1/2 Activation in Neuron Cultures by Citrus Extracts
3.2. Identification of the Components from Citrus grandis Peel
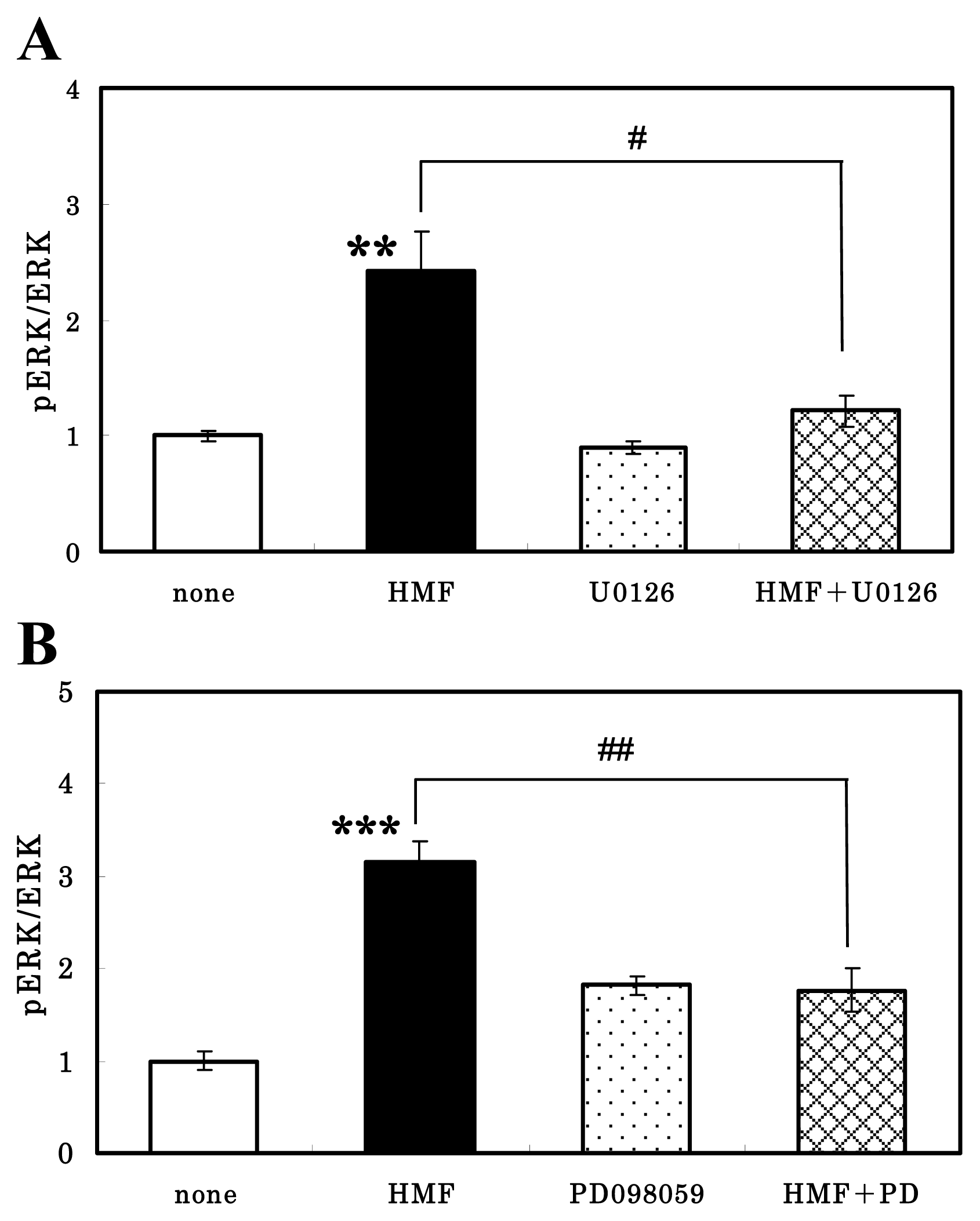
3.3. HMF-Mediated ERK1/2 Activation in Neuron Cultures
3.4. CREB Activation of Neuron Cultures by HMF
3.5. Enhancement of Memory by HMF
4. Conclusions
Acknowledgments
References
- Benavente-García, O.; Castillo, J.; Martín, F.R.; Ortuño, A.; del Rio, J.A. Uses and properties of Citrus flavonoids. J. Agric. Food Chem 1997, 45, 4505–4515. [Google Scholar]
- Benavente-García, O.; Castillo, J. Update on uses and properties of Citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem 2008, 56, 6185–6205. [Google Scholar]
- Spencer, J.P.E.; Vauzour, D.; Rendeiro, C. Flavonoids and cognition: The molecular mechanisms underlying their behavioural effects. Arch. Biochem. Biophys 2009, 492, 1–9. [Google Scholar]
- Maher, P.; Akaishi, T.; Abe, K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA 2006, 103, 16568–16573. [Google Scholar]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring β-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci. Lett 2006, 400, 230–234. [Google Scholar]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shi, R.-W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a Citrus flavonoid, improves memory impairment and Aβ pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther 2008, 326, 739–744. [Google Scholar]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Yamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Biophys. Res. Commun 2005, 337, 1330–1336. [Google Scholar]
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Song, S.-Y.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a Citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. J. Pharmacol. Sci 2007, 105, 122–126. [Google Scholar]
- Yamamoto, Y.; Shioda, N.; Han, F.; Moriguchui, S.; Nakajima, A.; Yokosuka, A.; Mimaki, Y.; Sashida, Y.; Yamakuni, T.; Ohizumi, Y.; et al. Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMK II and CREB phosphorylation. Brain Res 2009, 1295, 218–229. [Google Scholar]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of flavonoid constituents in Citrus fruits. J. Agric. Food Chem 1999, 47, 3565–3571. [Google Scholar]
- Samuels, I.S.; Karlo, J.C.; Faruzzi, A.N.; Pickering, K.; Herrup, K.; Sweatt, J.D.; Saitta, S.C.; Landreth, G.E. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J. Neurosci 2008, 28, 6983–6995. [Google Scholar]
- Impey, S.; Mark, M.; Vilacres, E.C.; Poser, S.; Yano, S.; Wayman, G.; Deloulme, J.C.; Chan, G.; Storm, D.R. Crosstalk between ERK and PKA is required for calcium stimulated of CREB-dependent transcription and ERK nuclear translocation. Neuron 1998, 21, 869–883. [Google Scholar]
- Tao, X.; Finkbeiner, S.; Amold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF synthesis by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar]
- Okada, M.; Makino, A.; Nakajima, M.; Okuyama, S.; Furukawa, S.; Furukawa, Y. Estrogen stimulates proliferation and differentiation of neural stem/progenitor cells through different signal transduction pathways. Int. J. Mol. Sci 2010, 11, 4114–4123. [Google Scholar]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar]
- Lin, N.; Sato, T.; Takayama, Y.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Novel anti-inflammatory actions of nobiletin, a Citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem. Pharmacol 2003, 65, 2065–2071. [Google Scholar]
- Cui, Y.; Wu, J.; Jung, S.-C.; Park, D.-B.; Maeng, Y.-H.; Hong, J.H.; Kim, S.-J.; Lee, S.-R.; Kim, S.-J.; Kim, S.J.; et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull 2010, 33, 1814–1821. [Google Scholar]
- Miyamoto, S.; Yasui, Y.; Tanaka, T.; Ohigashi, H.; Murakami, A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis 2008, 29, 1057–1063. [Google Scholar]
- Yi, L.T.; Xu, H.L.; Feng, J.; Zhan, X.; Zhou, L.P.; Cui, C.C. Involvement of monoaminergic systems in the antidepressant-like effect of nobiletin. Physiol. Behav 2011, 102, 1–6. [Google Scholar]
- Ito, C.; Fujiwara, K.; Koizumi, M.; Furukawa, H. Isolation and characterization of an antibacterial substance from Citrus plant. J. Chin. Chem. Soc 1998, 45, 89–91. [Google Scholar]
- Iwase, Y.; Takemura, Y.; Ju-ichi, M.; Yano, M.; Ito, C.; Furukawa, H.; Mukainaka, T.; Kuchide, M.; Tokuda, H.; Nishino, H. Cancer chemopreventive activity of 3,5,6,7,8,3′,4′-heptamethoxyflavone from the peel of citrus plants. Cancer Lett 2001, 163, 7–9. [Google Scholar]
- Manthey, J.A.; Bendele, P. Anti-inflammatory activity of an orange peel polymethoxylated flavone, 3,5,6,7,8,3′,4′-heptamethoxyflavone, in the rat carrageenan/paw edema and mouse lipopolysaccharide-challenge assays. J. Agric. Food Chem 2008, 56, 9399–9403. [Google Scholar]
- Murakami, A.; Matsumoto, K.; Koshimizu, K.; Ohigashi, H. Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide- and interferon-γ-induced IκB degradation on RAW264.7 macrophages. Cancer Lett 2003, 195, 17–25. [Google Scholar]
- Curini, M.; Carvotto, G.; Epifano, F.; Giannone, G. Chemistry and biological activity of natural and synthetic prenyloxycoumarins. Curr. Med. Chem 2006, 13, 199–222. [Google Scholar]
- Samuels, I.S.; Karlo, J.C.; Faruzzi, A.N.; Pickering, K.; Herrup, K.; Sweatt, J.D.; Saitta, S.C.; Landreth, G.E. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J. Neurosci 2008, 28, 6983–6995. [Google Scholar]
- Liu, Y.Z.; Boxer, D.S.; Latchman, D.S. Activation of the Bcl-2 promoter by nerve growth factor is mediated by the p42/p44 MAPK cascade. Nucleic Acids Res 1999, 27, 2086–2090. [Google Scholar]
- Nagase, H.; Yamakuni, T.; Matsuzaki, K.; Maruyama, Y.; Kasahara, J.; Hinohara, Y.; Kondo, S.; Mimaki, Y.T.; Sashida, Y.; Tank, A.W.; et al. Mechanism of neurotrophic action of nobiletin in PC12D cells. Biochemistry 2005, 44, 13683–13691. [Google Scholar]
- Al Rahim, M.; Nakajima, A.; Saigusa, D.; Tetsu, A.; Maruyama, Y.; Shibuya, M.; Yamakoshi, H.; Tomioka, Y.; Iwabuchi, Y.; Ohizumi, Y.; et al. 4′-Demethylnobiletin, a bioactive metabolite of nobiletin enhancing PKA/ERK/CREB signalling, rescues learning impairment associated with NMDA receptor antagonism via stimulation of the ERK cascade. Biochemistry 2009, 48, 7713–7721. [Google Scholar]
- Adams, J.P.; Sweatt, J.D. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu. Rev. Pharmacol. Toxicol 2002, 42, 135–163. [Google Scholar]
- Guscott, M.R.; Clarke, H.F.; Murray, F.; Grimwood, S.; Bristow, L.J.; Hutson, P.H. The effect of (±)-CP-101,606, an NMDA receptor NR2B subunit selective antagonist, in the Morris watermaze. Eur. J. Pharmacol 2003, 476, 193–199. [Google Scholar]





| Scientific Name | Conventional Name | Activity |
|---|---|---|
| Citrus unshiu | Unshu mikan | + |
| Citrus reticulate | Imadsu Ponkan | + |
| Citrus reticulata | Mandarin Orange (Kara) | + |
| Citrus iyo | Miyauchi Iyo | − |
| Citrus sinensis | Blood Orange (moro) | + |
| Citrus sinensis | Blood Orange (tarocco) | ± |
| Citrus grandis | Kawachi bankan | ++ |
| Citrus depressa | Hay Flat Lemon | + |
| 0.1% DMSO | − |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Furukawa, Y.; Okuyama, S.; Amakura, Y.; Watanabe, S.; Fukata, T.; Nakajima, M.; Yoshimura, M.; Yoshida, T. Isolation and Characterization of Activators of ERK/MAPK from Citrus Plants. Int. J. Mol. Sci. 2012, 13, 1832-1845. https://doi.org/10.3390/ijms13021832
Furukawa Y, Okuyama S, Amakura Y, Watanabe S, Fukata T, Nakajima M, Yoshimura M, Yoshida T. Isolation and Characterization of Activators of ERK/MAPK from Citrus Plants. International Journal of Molecular Sciences. 2012; 13(2):1832-1845. https://doi.org/10.3390/ijms13021832
Chicago/Turabian StyleFurukawa, Yoshiko, Satoshi Okuyama, Yoshiaki Amakura, Sono Watanabe, Takahiro Fukata, Mitsunari Nakajima, Morio Yoshimura, and Takashi Yoshida. 2012. "Isolation and Characterization of Activators of ERK/MAPK from Citrus Plants" International Journal of Molecular Sciences 13, no. 2: 1832-1845. https://doi.org/10.3390/ijms13021832





