Yeast Mitochondrial Interactosome Model: Metabolon Membrane Proteins Complex Involved in the Channeling of ADP/ATP
Abstract
:1. Introduction
2. The Different Proteins Involved in the Mitochondrial Membrane Transport of Adenine Nucleotides
2.1. The ADP/ATP Carrier
2.1.1. Overview
2.1.2. Oligomeric State of the ADP/ATP Carrier
2.1.3. The ADP/ATP Carrier of S. Cerevisiae
2.1.4. Human Pathophysiological Aspects
2.2. The Inorganic Phosphate Carrier—PiC
2.2.1. Discovery and Biochemical Properties of PiC
2.2.2. Transport Mechanism of Mitochondrial PiC
2.3. The Mitochondrial Porin
2.3.1. Identification and Physiological Characteristics of Porin
2.3.2. High-Resolution 3D Structures of Porin
2.3.3. Selectivity of the Pore
2.3.3.1. Nucleotide Binding and Derived Molecules
2.3.3.2. Binding of Ca2+
2.3.3.3. Potential Partners of the Porin
2.3.4. Supra-Molecular Organization of Porin in the Membrane
2.3.5. Role of Lipids in Porin Activities
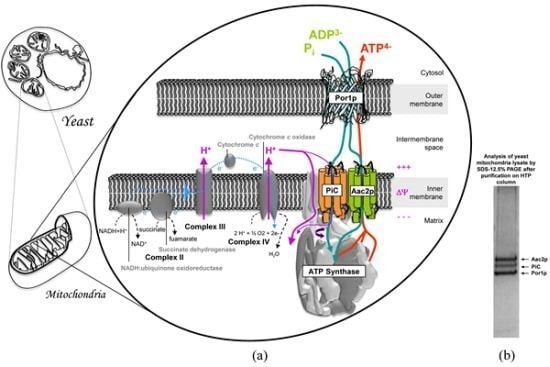
3. Mitochondrial Interactosome Model
4. Conclusions
Acknowledgements
References
- Clémençon, B.; Rey, M.; Dianoux, A.C.; Trézéguet, V.; Lauquin, G.J.; Brandolin, G.; Pelosi, L. Structure-function relationships of the C-terminal end of the Saccharomyces cerevisiae ADP/ATP carrier isoform 2. J. Biol. Chem 2008, 283, 11218–11225. [Google Scholar]
- Clémençon, B.; Rey, M.; Trézéguet, V.; Forest, E.; Pelosi, L. Yeast ADP/ATP carrier isoform 2: Conformational dynamics and role of the RRRMMM signature sequence methionines. J. Biol. Chem 2011, 286, 36119–361131. [Google Scholar]
- Rey, M.; Man, P.; Clémençon, B.; Trézéguet, V.; Brandolin, G.; Forest, E.; Pelosi, L. Conformational dynamics of the bovine mitochondrial ADP/ATP carrier isoform 1 revealed by hydrogen/deuterium exchange coupled to mass spectrometry. J. Biol. Chem 2010, 285, 34981–34990. [Google Scholar]
- Verkman, A.S. Solute and macromolecule diffusion in cellular aqueous compartments. Trends Biochem. Sci 2002, 27, 27–33. [Google Scholar]
- Azimi, M.; Jamali, Y.; Mofrad, M.R.K. Accounting for diffusion in agent based models of reaction-diffusion systems with application to cytoskeletal diffusion. PLoS One 2011, 6, e25306:1–e25306:9. [Google Scholar]
- Medalia, O.; Weber, I.; Frangakis, A.S.; Nicastro, D.; Gerisch, G.; Baumeister, W. Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science 2002, 298, 599–620. [Google Scholar]
- Zimmermann, S.B.; Trach, S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol 1991, 222, 1209–1213. [Google Scholar]
- Scalettar, B.A.; Abney, J.R.; Hackenbrock, C.R. Dynamics, structure, and function are coupled in the mitochondrial matrix. Proc. Natl. Acad. Sci. USA 1991, 88, 8057–8061. [Google Scholar]
- Dix, J.A.; Verkman, A.S. Crowding effects on diffusion in solutions and cells. Annu. Rev. Biophys 2008, 37, 247–263. [Google Scholar]
- Saks, V.A.; Khuchua, Z.A.; Vasilyeva, E.V.; Yu Belikova, O.; Kuznetsov, A. Metabolic compartimentation and substrate channeling in muscle cells. Role of coupled creatine kinases in vivo regulation of cellular respiration—A synthesis. Mol. Cell. Biochem 1994, 133, 155–192. [Google Scholar]
- Goobes, R.; Kahana, N.; Cohen, O.; Minsky, A. Metabolic buffering exerted by macromolecular crowding on DNA-DNA interactions: Origin and physiological significance. Biochemistry 2003, 42, 2431–2440. [Google Scholar]
- Wieczorek, G.; Zielenkiewicz, P. Influence of macromolecular crowding on protein-protein association rates—A Brownian dynamics study. Biophys. J 2008, 95, 5030–5036. [Google Scholar]
- Zimmerman, S.B.; Harrison, B. Macromolecular crowding increases binding of DNA polymerase to DNA: An adaptive effect. Biochemistry 1987, 84, 1871–1875. [Google Scholar]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem 2001, 276, 10577–10580. [Google Scholar]
- van den Berg, B.; Ellis, R.J.; Dobson, C.M. Effects of macromolecular crowding on protein folding and aggregation. EMBO J 1999, 24, 6927–6933. [Google Scholar]
- van den Berg, B.; Wain, R.; Dobson, C.M.; Ellis, R.J. Macromolecular crowding perturbs protein refolding kinetics: Implications for folding inside the cell. EMBO J 2000, 19, 3870–3875. [Google Scholar]
- Galan, A.; Sot, B.; Llorca, O.; Carrascosa, J.L.; Valpuesta, J.M.; Muga, A. Excluded volume effects on the refolding and assembly of an oligomeric protein. GroEL, a case study. J. Biol. Chem 2001, 276, 957–964. [Google Scholar]
- Dong, H.; Qin, S.; Zhou, H.X. Effects of macromolecular crowding on protein conformational changes. PLoS Comput. Biol 2010, 6, e1000833:1–e1000833:10. [Google Scholar]
- Welch, G.R. On the role of organized multienzyme systems in cellular metabolism: A general synthesis. Prog. Biophys. Mol. Biol 1977, 32, 103–191. [Google Scholar]
- Tompa, P.; Batke, J.; Ovàdi, J. How to determine the efficiency of intermediate transfer in an interacting enzyme system? FEBS Lett 1987, 214, 244–248. [Google Scholar]
- Ovàdi, J.; Saks, V. On the origin of intracellular compartmentation and organized metabolic systems. Mol. Cell. Biochem 2004, 256–257, 5–12. [Google Scholar]
- Philbert, J. One and half century of diffusion: Fick, Einstein, before and beyond. Diffus. Fundam 2006, 4, 6:1–6:19. [Google Scholar]
- Srivastava, D.K.; Bernhard, S.A. Metabolite transfert via enzyme-enzyme complexes. Science 1986, 234, 1081–1086. [Google Scholar]
- Huang, X.; Holden, H.M.; Raushel, F.M. Channeling of substrates and intermediates in enzyme-catalyzed reactions. Annu. Rev. Biochem 2001, 70, 149–180. [Google Scholar]
- van Noorden, C.J.; Jonges, G.N. Analysis of enzyme reactions in situ. Histochem J 1995, 27, 101–118. [Google Scholar]
- Boonacker, E.; Stap, J.; Koehler, A.; van Noorden, C.J. The need for metabolic mapping in living cells and tissues. Acta Histochem. 2004, 106, 89–96. [Google Scholar]
- Srere, P.A. The metabolon. Trends Biochem. Sci 1985, 10, 109–110. [Google Scholar]
- Srere, P.A. Complexes of sequential metabolic enzymes. Annu. Rev. Biochem 1987, 56, 89–124. [Google Scholar]
- Vélot, C.; Mixon, M.B.; Teige, M.; Srere, P.A. Model of a quinary structure between krebs TCA cycle enzymes: A model for the metabolon. Biochemistry 1997, 36, 14271–14276. [Google Scholar]
- Clarke, F.M.; Masters, C.J. On the association of glycolytic enzymes with structural proteins of skeletal muscles. Biochim. Biophys 1975, 381, 37–46. [Google Scholar]
- Ottaway, J.H.; Mowbray, J. The role of compartmentation in the control of glycolysis. Curr. Top. Cell. Regul 1977, 12, 107–208. [Google Scholar]
- Kurganov, B.I.; Sugrobova, N.P.; Mil’man, L.S. Supramolecular organization of glycolytic enzymes. J. Theor. Biol. 1985, 116, 509–526. [Google Scholar]
- Brooks, S.P.; Storey, K.B. The effect of enzyme-enzyme complexes on the overall glycolytic rate in vivo. Biochem. Int 1991, 25, 477–489. [Google Scholar]
- Maughan, D.W.; Henkin, J.A.; Vigoreaux, J.O. Concentrations of glycolytic enzymes and other cytosolic proteins in the diffusible fraction of a vertebrate muscle proteome. Mol. Cell Proteomics 2005, 10, 1541–1549. [Google Scholar]
- Masters, C. Interactions between glycolytic enzymes and components of the cytomatrix. J. Cell Biol 1984, 99, 222–225. [Google Scholar]
- Masters, C.J.; Reid, S.; Don, M. Glycolysis—New concepts in an old pathway. Mol. Cell Biochem 1987, 76, 3–14. [Google Scholar]
- Volker, K.W.; Reinitz, C.A.; Knull, H.R. Glycolytic enzymes and assembly of microtubule networks. Comp. Biochem. Physiol. B: Biochem. Mol. Biol 1995, 112, 503–514. [Google Scholar]
- Vertessy, B.G.; Kovacs, J.; Low, P.; Lehotzky, A.; Molnar, A.; Orosz, F.; Ovadi, J. Characterization of microtubule-phosphofructokinase complex: Specific effects of MgATP and vinblastine. Biochemistry 1997, 36, 2051–2062. [Google Scholar]
- Waingeh, V.F.; Gustafson, C.D.; Kozliak, E.I.; Lowe, S.L.; Knull, H.R.; Thomasson, K.A. Glycolytic enzyme interactions with yeast and skeletal muscle F-actin. Biophys. J 2006, 90, 1371–1384. [Google Scholar]
- Vélot, C.; Mixon, M.B.; Teige, M.; Srere, P.A. Model of a quinary structure between Krebs TCA cycle enzymes: A model for the metabolon. Biochemistry 1997, 25, 14271–14276. [Google Scholar]
- Rajakumari, S.; Daum, G. Multiple functions as lipase, steryl ester hydrolase, phospholipase, and acyltransferase of Tgl4p from the yeast Saccharomyces cerevisiae. J. Biol. Chem 2010, 285, 15769–15776. [Google Scholar]
- Guelzim, N.; Mariotti, F.; Martin, P.G.; Lasserre, F.; Pineau, T.; Hermier, D. A role for PPARα in the regulation of arginine metabolism and nitric oxide synthesis. Amino Acids 2011, 41, 969–979. [Google Scholar]
- Bhaskaran, H.; Perona, J.J. Two-step aminoacylation of tRNA without channeling in Archaea. J. Mol. Biol 2011, 411, 854–869. [Google Scholar]
- Ko, Y.H.; Delannoy, M.; Hullihen, J.; Chiu, W.; Pedersen, P.L. Mitochondrial ATP synthasome cristae-enriched membranes and a multiwall detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. J. Biol. Chem 2003, 278, 12305–12309. [Google Scholar]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar]
- Mitchell, P. Compartimentation and communication in living systems ligand conduction: General catalytic principle in chemical osmotic and chemiosmotic reaction systems. Eur. J. Biochem 1979, 95, 1–20. [Google Scholar]
- Saks, V. Integrated and Organized Cellular Energetic Systems: Maxwell’s Demon and Organized Cellular Metabolism. In Molecular System Bioenergetics. Energy for Life; Saks, V., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 94–96. [Google Scholar]
- Saks, V.; Guzun, R.; Timohhina, N.; Tepp, K.; Varikmaa, M.; Monge, C; Beraud, N.; Kaambre, T.; Kuznetsov, A.; Kadaja, L.; Eimre, M.; Seppet, E. Structure-function relationships in feedback regulation of energy fluxes in vivo in health and disease: Mitochondrial Interatosome. Biochim. Biophys. Acta 2010, 1797, 678–697. [Google Scholar]
- Pfaff, E.; Klingenberg, M.; Heldt, H.W. Unspecific permeation and specific exchange of adenine nucleotides in liver mitochondria. Biochim. Biophys. Acta 1965, 104, 312–315. [Google Scholar]
- Duee, E.D.; Vignais, P.V. Exchange between extra- and intramitochondrial adenine nucleotides. Biochim. Biophys. Acta 1965, 107, 184–188. [Google Scholar]
- Klingenberg, M.; Pfaff, E. Metabolic control in mitochondria by adenine nucleotide translocation. Biochem. Soc. Symp 1968, 27, 105–122. [Google Scholar]
- Vignais, P.V. The mitochondrial adenine nucleotide translocator. J. Bioenerg 1976, 8, 9–17. [Google Scholar]
- Klingenberg, M. The ADP-ATP translocation in mitochondria, a membrane potential controlled transport. J. Membr. Biol 1980, 56, 97–105. [Google Scholar]
- LaNoue, K.; Mizani, S.M.; Klingenberg, M. Electrical imbalance of adenine nucleotide transport across the mitochondrial membrane. J. Biol. Chem 1978, 253, 191–198. [Google Scholar]
- Duszynski, J.; Bogucka, K.; Letko, G.; Kuster, U.; Kunz, W.; Wojtczak, L. Relationship between the energy cost of ATP transport and ATP synthesis in mitochondria. Biochim. Biophys. Acta 1981, 637, 217–223. [Google Scholar]
- Kramer, R.; Klingenberg, M. Modulation of the reconstituted adenine nucleotide exchange by membrane potential. Biochemistry 1980, 19, 556–560. [Google Scholar]
- Pebay-Peyroula, E.; Dahout-Gonzalez, C.; Kahn, R.; Trezeguet, V.; Lauquin, G.J.; Brandolin, G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 2003, 426, 39–44. [Google Scholar]
- Robinson, A.J.; Kunji, R.S. Mitochondrial carries in the cytoplasmic state have a common substrate binding site. Proc. Natl. Acad. Sci. USA 2006, 103, 2617–2622. [Google Scholar]
- Berardi, M.J.; Shih, W.M.; Harrison, S.C.; Chou, J.J. Mitochondrial uncoupling protein 2 structure determined by NMR molecular fragment searching. Nature 2011, 476, 109–113. [Google Scholar]
- Riccio, P.; Aquila, H.; Klingenberg, M. Purification of the carboxy-atractylate binding protein from mitochondria. FEBS Lett 1975, 56, 133–138. [Google Scholar]
- Aquila, H.; Eiermann, W.; Babel, W.; Klingenberg, M. Isolation of the ADP/ATP translocator from beef heart mitochondria as the bongkrekate-protein complex. Eur. J. Biochem 1978, 85, 549–560. [Google Scholar]
- Hackenberg, H.; Klingenberg, M. Molecular weight and hydrodynamic parameters of the adenosine 5′-diphosphate-adenosine 5′-triphosphate carrier in Triton X-100. Biochemistry 1980, 19, 548–555. [Google Scholar]
- Block, M.R.; Zaccai, G.; Lauquin, G.J.; Vignais, P.V. Small angle neutron scattering of the mitochondrial ADP/ATP carrier protein in detergent. Biochem. Biophys. Res. Commun 1882, 109, 471–477. [Google Scholar]
- Schroers, A.; Burkovski, A.; Wohlrab, H; Kramer, R. The phosphate carrier from yeast mitochondria. Dimerization is a prerequisite for function. J. Biol. Chem. 1998, 273, 14269–14276. [Google Scholar]
- Kotaria, R.; Mayor, J.A.; Walters, D.E.; Kaplan, R.S. Oligomeric state of wild-type and cysteine-less yeast mitochondrial citrate transport proteins. J. Bioenerg. Biomembr 1999, 31, 543–549. [Google Scholar]
- Brandolin, G.; Dupont, Y.; Vignais, P.V. Exploration of the nucleotide binding sites of the isolated ADP/ATP carrier protein from beef heart mitochondria. 2. Probing of the nucleotide sites by formycin triphosphate, a fluorescent transportable analogue of ATP. Biochemistry 1982, 21, 6348–6353. [Google Scholar]
- Block, M.R.; Vignais, P.V. Substrate-site interactions in the membrane-bound adenine-nucleotide carrier as disclosed by ADP and ATP analogs. Biochim. Biophys. Acta 1984, 767, 369–376. [Google Scholar]
- Nury, H.; Dahout-Gonzalez, C.; Trezeguet, V.; Lauquin, G.; Brandolin, G.; Pebay-Peyroula, E. Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett 2005, 579, 6031–6036. [Google Scholar]
- Nury, H.; Manon, F.; Arnou, B.; le Maire, M.; Pebay-Peyroula, E.; Ebel, C. Mitochondrial bovine ADP/ATP carrier in detergent is predominantly monomeric but also forms multimeric species. Biochemistry 2008, 47, 12319–12331. [Google Scholar]
- Bamber, L.; Harding, M.; Butler, P.J.; Kunji, E.R. Yeast mitochondrial ADP/ATP carriers are monomeric in detergents. Proc. Natl. Acad. Sci. USA 2006, 103, 16224–16229. [Google Scholar]
- Bamber, L.; Harding, M.; Monne, M.; Slotboom, D.J.; Kunji, E.R. The yeast mitochondrial ADP/ATP carrier functions as a monomer in mitochondrial membranes. Proc. Natl. Acad. Sci. USA 2007, 104, 10830–10834. [Google Scholar]
- Bamber, L.; Slotboom, D.J.; Kunji, E.R. Yeast mitochondrial ADP/ATP carriers are monomeric in detergents as demonstrated by differential affinity purification. J.Mol. Biol 2007, 371, 388–395. [Google Scholar]
- Lauquin, G.; Lunardi, J.; Vignais, P.V. Effect of genetic and physiological manipulations on the kinetic and binding parameters of the adenine nucleotide translocator in Saccharomyces cervisiae and Candida utilis. Biochimie 1976, 58, 1213–1220. [Google Scholar]
- Gavurnikova, G.; Sabova, L.; Kissova, I.; Haviernik, P.; Kolarov, J. Transcription of the AAC1 gene encoding an isoform of mitochondrial ADP/ATP carrier in Saccharomyces cerevisiae is regulated by oxygen in a heme-independent manner. Eur. J. Biochem 1996, 239, 759–763. [Google Scholar]
- Lawson, J.E.; Douglas, M.G. Separate genes encode functionally equivalent ADP/ATP carrier proteins in Saccharomyces cerevisiae. Isolation and analysis of AAC2. J. Biol. Chem 1988, 263, 14812–14818. [Google Scholar]
- Drgon, T.; Sabova, L.; Nelson, N.; Kolarov, J. ADP/ATP translocator is essential only for anaerobic growth of yeast Saccharomyces cerevisiae. FEBS Lett 1991, 289, 159–162. [Google Scholar]
- Kolarov, J.; Kolarova, N.; Nelson, N. A third ADP/ATP translocator gene in yeast. J. Biol. Chem 1990, 265, 12711–12716. [Google Scholar]
- Sabova, L.; Zeman, I.; Supek, F.; Kolarov, J. Transcriptional control of AAC3 gene encoding mitochondrial ADP/ATP translocator in Saccharomyces cerevisiae by oxygen, heme and ROX1 factor. Eur. J. Biochem 1993, 213, 547–553. [Google Scholar]
- Stepien, G.; Torroni, A.; Chung, A.B.; Hodge, J.A.; Wallace, D.C. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J. Biol. Chem 1992, 267, 14592–14597. [Google Scholar]
- Chevrollier, A.; Loiseau, D.; Chabi, B.; Renier, G.; Douay, O.; Malthièry, Y.; Stepien, G. ANT2 isoform required for cancer cell glycolysis. J. Bioenerg. Biomembr 2005, 35, 307–316. [Google Scholar]
- Dolce, V.; Scarcia, P.; Iacopetta, D.; Palmieri, F. A fourth ADP/ATP carrier isoform in man: Identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett 2005, 579, 633–637. [Google Scholar]
- Trézéguet, V.; Pélosi, L.; Lauquin, G.J.; Brandolin, G. The mitochondrial ADP/ATP carrier: Functional and structural studies in the route of elucidating pathophysiological aspects. J. Bioenerg. Biomembr 2008, 40, 435–443. [Google Scholar]
- Wohlrab, H. Purification and reconstitution of the mitochondrial phosphate transporter. Ann. N. Y. Acad. Sci 1980, 358, 364–367. [Google Scholar]
- Wohlrab, H. Molecular aspects of inorganic phosphate transport in mitochondria. Biochim. Biophys. Acta 1986, 853, 115–134. [Google Scholar]
- Kramer, R.; Palmieri, F. Molecular aspects of isolated and reconstituted carrier proteins from animal mitochondria. Biochim. Biophys. Acta 1989, 974, 1–23. [Google Scholar]
- Wehrle, J.P.; Pedersen, P.L. Phosphate transport processes in eukaryotic cells. J. Membr. Biol 1989, 111, 199–213. [Google Scholar]
- Aquila, H.; Link, T.A.; Klingenberg, M. Solute carriers involved in energy transfer of mitochondria form a homologous protein family. FEBS Lett 1987, 212, 1–9. [Google Scholar]
- Walker, J.E.; Runswick, M.J. The mitochondrial transport protein superfamily. J. Bioenerg. Biomembr 1993, 25, 435–446. [Google Scholar]
- Phelps, A.; Schobert, C.T.; Wohlrab, H. Cloning and characterization of the mitochondrial phosphate transport protein gene from the yeast Saccharomyces cerevisiae. Biochemistry 1991, 30, 248–252. [Google Scholar]
- Zara, V.; Dietmeier, K.; Palmisano, A.; Vozza, A.; Rassow, J.; Palmieri, F.; Pfanner, N. Yeast mitochondria laking the phosphate carrier/p32 are blocked in phosphate transport but can import preproteins after regeneration of a membrane potential. Mol. Cell. Biol 1996, 16, 6524–6531. [Google Scholar]
- Aquila, H.; Link, T.A.; Klingenberg, M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J 1985, 4, 2369–2376. [Google Scholar]
- Kuan, J.; Saier, M.H., Jr. The mitochondrial carrier family of transport proteins: Structural, functional, and evolutionary relationships. Crit. Rev. Biochem. Mol. Biol. 1993, 28, 209–233. [Google Scholar]
- Stappen, R.; Kramer, R. Functional properties of the reconstituted phosphate carrier from bovine heart mitochondria: Evidence for asymmetric orientation and characterization of three different transport modes. Biochim. Biophys. Acta 1993, 1149, 40–48. [Google Scholar]
- Phelps, A.; Briggs, C.; Mincone, L.; Wohlrab, H. Mitochondrial phosphate transport protein. replacements of glutamic, aspartic, and histidine residues affect transport and protein conformation and point to a coupled proton transport path. Biochemistry 1996, 35, 10757–10762. [Google Scholar]
- Stappen, R.; Kramer, R. Kinetic mechanism of phosphate/phosphate and phosphate/OH– antiports catalyzed by reconstituted phosphate carrier from beef heart mitochondria. J. Biol. Chem 1994, 269, 11240–11246. [Google Scholar]
- Herick, K.; Stappen, R.; Krämer, R. Comparaison of Functional and Structural Aspects of the Reconstituted Phosphate and Aspartate/Glutamate Carrier from Mitochondria. In Thirty Years of Progress in Mitochondrial Bioenergetics and Molecular Biology; Palmieri, F., Ed.; Elsevier Science Publishers B.V: Amsterdam, The Netherlands, 1995; pp. 83–87. [Google Scholar]
- Schroers, A.; Kramer, R.; Wohlrab, H. The reversible antiport-uniport conversion of the phosphate carrier from yeast mitochondria depends on the presence of a single cysteine. J. Biol. Chem 1997, 272, 10558–10564. [Google Scholar]
- Zackova, M.; Kramer, R.; Jezek, P. Interaction of mitochondrial phosphate carrier with fatty acids and hydrophobic phosphate analogs. Int. J. Biochem. Cell Biol 2000, 32, 499–508. [Google Scholar]
- Wohlrab, H.; Briggs, C. Yeast mitochondrial phosphate transport protein expressed in Escherichia coli. Site-directed mutations at threonine-43 and at a similar location in the second tandem repeat (isoleucine-141). Biochemistry 1994, 33, 9371–9375. [Google Scholar]
- Schroers, A.; Burkovski, A.; Wohlrab, H.; Kramer, R. The phosphate carrier from yeast mitochondria. Dimerization is a prerequisite for function. J. Biol. Chem 1998, 273, 14269–14276. [Google Scholar]
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 2008, 1778, 1978–2021. [Google Scholar]
- Mayr, J.A.; Merkel, O.; Kohlwein, S.D.; Gebhardt, B.R.; Böhles, H; Fötschl, U.; Koch, J.; Jaksch, M.; Lochmüller, H; Horvath, R.; Freisinger, P.; Sperl, W. Mitochondrial phosphate-carrier deficiency: A novel disorder of oxidative phosphorylation. Am. J. Hum. Genet. 2007, 80, 478–489. [Google Scholar]
- Colombini, M. Voltage gating in the mitochondrial channel, VDAC. J. Membr. Biol 1989, 111, 103–111. [Google Scholar]
- Forte, M.; Guy, H.R.; Mannella, C.A. Molecular genetics of the VDAC ion channel: Structural model and sequence analysis. J. Bioenerg. Biomembr 1987, 19, 341–350. [Google Scholar]
- Mannella, C.A.; Bonner, W.D., Jr. Biochemical characteristics of the outer membranes of plant mitochondria. Biochim. Biophys. Acta 1975, 413, 213–225. [Google Scholar]
- Schein, S.J.; Colombini, M.; Finkelstein, A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J. Membr. Biol 1976, 30, 99–120. [Google Scholar]
- Colombini, M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature 1979, 279, 643–645. [Google Scholar]
- de Pinto, V.; Ludwig, O.; Krause, J.; Benz, R.; Palmieri, F. Porin pores of mitochondrial outer membranes from high and low eukaryotic cells: Biochemical and biophysical characterization. Biochim. Biophys. Acta 1987, 894, 109–119. [Google Scholar]
- Colombini, M. VDAC: The channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem 2004, 256–257, 107–115. [Google Scholar]
- Mannella, C.A. Conformational changes in the mitochondrial channel protein, VDAC, and their functional implications. J. Struct. Biol 1998, 121, 207–218. [Google Scholar]
- Shoshan-Barmatz, V.; Gincel, D. The voltage-dependent anion channel: Characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem. Biophys 2003, 39, 279–292. [Google Scholar]
- Liu, M.Y.; Colombini, M. Regulation of mitochondrial respiration by controlling the permeability of the outer membrane through the mitochondrial channel, VDAC. Biochim. Biophys. Acta 1992, 1098, 255–260. [Google Scholar]
- Lemeshko, S.V.; Lemeshko, V.V. Metabolically derived potential on the outer membrane of mitochondria: A computational model. Biophys. J 2000, 79, 2785–2800. [Google Scholar]
- Lemeshko, S.V.; Lemeshko, V.V. Energy flux modulation on the outer membrane of mitochondria by metabolically-derived potential. Mol. Cell. Biochem 2004, 256–257, 127–139. [Google Scholar]
- Blachly-Dyson, E.; Song, J.; Wolfgang, W.J.; Colombini, M.; Forte, M. Multicopy suppressors of phenotypes resulting from the absence of yeast VDAC encode a VDAC-like protein. Mol. Cell. Biol 1997, 17, 5727–5738. [Google Scholar]
- Lee, A.C.; Xu, X.; Blachly-Dyson, E.; Forte, M.; Colombini, M. The role of yeast VDAC genes on the permeability of the mitochondrial outer membrane. J. Membr. Biol 1998, 161, 173–181. [Google Scholar]
- Dihanich, M.; Suda, K.; Schatz, G. A yeast mutant lacking mitochondrial porin is respiratory-deficient, but can recover respiration with simultaneous accumulation of an 86-KD extramitochondrial protein. EMBO J 1987, 6, 723–728. [Google Scholar]
- Michejda, J.; Guo, X.J.; Lauquin, G.J. The respiration of cells and mitochondria of porin deficient yeast mutants is coupled. Biochem. Biophys. Res. Commun 1990, 171, 354–361. [Google Scholar]
- Bayrhuber, M.; Meins, T.; Habeck, M.; Becker, S.; Giller, K.; Villinger, S.; Vonrhein, C.; Griesinger, C.; Zweckstetter, M.; Zeth, K. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA 2008, 105, 15370–15375. [Google Scholar]
- Hiller, S.; Garces, R.G.; Malia, T.J.; Orekhov, V.Y.; Colombini, M.; Wagner, G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 2008, 321, 1206–1210. [Google Scholar]
- Ujwal, R.; Cascio, D.; Chaptal, V.; Ping, P.; Abramson, J. Crystal packing analysis of murine VDAC1 crystals in a lipidic environment reveals novel insights on oligomerization and orientation. Channels 2009, 3, 167–170. [Google Scholar]
- Abu-Hamad, S.; Arbel, N.; Calo, D.; Arzoine, L.; Israelson, A.; Keinan, N.; Ben-Romano, R.; Friedman, O.; Shoshan-Barmatz, V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci 2009, 122, 1906–1916. [Google Scholar]
- Colombini, M. VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta 2012, in press. [Google Scholar]
- Florke, H.; Thinnes, F.P.; Winkelbach, H.; Stadtmuller, U.; Paetzold, G.; Morys-Wortmann, C.; Hesse, D.; Sternbach, H.; Zimmermann, B.; Kaufmann-Kolle, P. Channel active mammalian porin, purified from crude membrane fractions of human B lymphocytes and bovine skeletal muscle, reversibly binds adenosine triphosphate (ATP). Biol. Chem. Hoppe Seyler 1994, 375, 513–520. [Google Scholar]
- Lee, A.C.; Zizi, M.; Colombini, M. β-NADH decreases the permeability of the mitochondrial outer membrane to ADP by a factor of 6. J. Biol. Chem 1994, 269, 30974–30980. [Google Scholar]
- Yehezkel, G.; Hadad, N.; Zaid, H.; Sivan, S.; Shoshan-Barmatz, V. Nucleotide-binding sites in the voltage-dependent anion channel: Characterization and localization. J. Biol. Chem 2006, 281, 5938–5946. [Google Scholar]
- Rostovtseva, T.; Colombini, M. VDAC channels mediate and gate the flow of ATP: Implications for the regulation of mitochondrial function. Biophys. J 1997, 72, 1954–1962. [Google Scholar]
- Rostovtseva, T.K.; Bezrukov, S.M. ATP transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis. Biophys. J 1998, 74, 2365–2373. [Google Scholar]
- Rostovtseva, T.K.; Komarov, A.; Bezrukov, S.M.; Colombini, M. VDAC channels differentiate between natural metabolites and synthetic molecules. J. Membr. Biol 2002, 187, 147–156. [Google Scholar]
- Rostovtseva, T.K.; Komarov, A.; Bezrukov, S.M.; Colombini, M. Dynamics of nucleotides in VDAC channels: Structure-specific noise generation. Biophys. J 2002, 82, 193–205. [Google Scholar]
- Yehezkel, G.; Hadad, N.; Zaid, H.; Sivan, S.; Shoshan-Barmatz, V. Nucleotide-binding sites in voltage-dependent anion channel: Characterization and localization. J. Biol. Chem 2006, 281, 5938–5946. [Google Scholar]
- Yehezkel, G.; Abu-Hamad, S.; Shoshan-Barmatz, V. An N-terminal nucleotide-binding site in VDAC1: Involvement in regulating mitochondrial function. J. Cell. Physiol 2007, 212, 551–561. [Google Scholar]
- Berridge, M.J.; Bootman, M.D.; Lipp, P. Calcium—A life and death signal. Nature 1998, 395, 645–648. [Google Scholar]
- Mooren, F.C.; Kinne, R.K. Cellular calcium in health and disease. Biochim. Biophys. Acta 1998, 1406, 127–151. [Google Scholar]
- Crompton, M.; Virji, S.; Doyle, V.; Johnson, N.; Ward, J.M. The mitochondrial permeability transition pore. Biochem. Soc. Symp 1999, 66, 167–179. [Google Scholar]
- Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J 1999, 341, 233–249. [Google Scholar]
- Pozzan, T.; Rizzuto, R. The renaissance of mitochondrial calcium transport. Eur. J. Biochem 2000, 267, 5269–5273. [Google Scholar]
- Shoshan-Barmatz, V.; Gincel, D. The voltage-dependent anion channel: Characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem. Biophys 2003, 39, 279–292. [Google Scholar]
- Israelson, A.; Abu-Hamad, S.; Zaid, H.; Nahon, E.; Shoshan-Barmatz, V. Localization of the voltage-dependent anion channel-1 Ca2+-binding sites. Cell Calcium 2006, 235–244. [Google Scholar]
- Nakashima, R.A.; Mangan, P.S.; Colombini, M.; Pedersen, P.L. Hexokinase receptor complex in hepatoma mitochondria: Evidence from N,N′-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry 1986, 25, 1015–1021. [Google Scholar]
- Al Jamal, J.A. Involvement of porin N,N-dicyclohexylcarbodiimide-reactive domain in hexokinase binding to the outer mitochondrial membrane. Protein J 2005, 24, 1–8. [Google Scholar]
- Zaid, H.; Abu-Hamad, S.; Israelson, A.; Nathan, I.; Shoshan-Barmatz, V. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ 2005, 12, 751–760. [Google Scholar]
- Abu-Hamad, S.; Zaid, H.; Israelson, A.; Nahon, E.; Shoshan-Barmatz, V. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: Mapping the site of binding. J. Biol. Chem 2008, 283, 13482–13490. [Google Scholar]
- Marzo, I.; Brenner, C.; Zamzami, N.; Susin, S.A.; Beutner, G.; Brdiczka, D.; Remy, R.; Xie, Z.H.; Reed, J.C.; Kroemer, G. The permeability transition pore complex: A target for apoptosis regulation by caspases and bcl-2-related proteins. J. Exp. Med 1998, 187, 1261–1271. [Google Scholar]
- Beutner, G.; Ruck, A.; Riede, B.; Brdiczka, D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim. Biophys. Acta 1998, 1368, 7–18. [Google Scholar]
- Halestrap, A.P.; Brenner, C. The adenine nucleotide translocase: A central component of the mitochondrial permeability transition pore and key player in cell death. Curr. Med. Chem 2003, 10, 1507–1525. [Google Scholar]
- Brdiczka, D.; Kaldis, P.; Wallimann, T. In vitro complex formation between the octamer of mitochondrial creatine kinase and porin. J. Biol. Chem 1994, 269, 27640–27644. [Google Scholar]
- Stachowiak, O.; Schlattner, U.; Dolder, M.; Wallimann, T. Oligomeric state and membrane binding behaviour of creatine kinase isoenzymes: Implications for cellular function and mitochondrial structure. Mol. Cell. Biochem 1998, 184, 141–151. [Google Scholar]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The “phosphocreatine circuit” for cellular energy homeostasis. Biochem. J 1992, 281, 21–40. [Google Scholar]
- Schlattner, U.; Dolder, M.; Wallimann, T.; Tokarska-Schlattner, M. Mitochondrial creatine kinase and mitochondrial outer membrane porin show a direct interaction that is modulated by calcium. J. Biol.Chem 2001, 276, 48027–48030. [Google Scholar]
- Tsujimoto, Y.; Shimizu, S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ 2000, 7, 1174–1181. [Google Scholar]
- Azoulay-Zohar, H.; Israelson, A.; Abu-Hamad, S.; Shoshan-Barmatz, V. In self-defence: Hexokinase promotes voltage-dependent anion channel closure and prevents mitochondriamediated apoptotic cell death. Biochem. J 2004, 377, 347–355. [Google Scholar]
- Malia, T.J.; Wagner, G. NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry 2007, 46, 514–525. [Google Scholar]
- Pastorino, J.G.; Hoek, J.B. Hexokinase II: The integration of energy metabolism and control of apoptosis. Curr. Med. Chem 2003, 10, 1535–1551. [Google Scholar]
- Carre, M.; Andre, N.; Carles, G.; Borghi, H.; Brichese, L.; Briand, C.; Braguer, D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J. Biol. Chem 2002, 277, 33664–33669. [Google Scholar]
- Monge, C.; Beraud, N.; Kuznetsov, A.V.; Rostovtseva, T.; Sackett, D.; Schlattner, U.; Vendelin, M.; Saks, V.A. Regulation of respiration in brain mitochondria and synaptosomes: Restrictions of ADP diffusion in situ, roles of tubulin, and mitochondrial creatine kinase. Mol. Cell. Biochem 2008, 318, 147–165. [Google Scholar]
- Rostovtseva, T.K.; Bezrukov, S.M. VDAC regulation: Role of cytosolic proteins and mitochondrial lipids. J. Bioenerg. Biomembr 2008, 40, 163–170. [Google Scholar]
- Rostovtseva, T.K.; Sheldon, K.L.; Hassanzadeh, E.; Monge, C.; Saks, V.; Bezrukov, S.M.; Sackett, D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; de Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol 2006, 175, 901–911. [Google Scholar]
- Boldogh, I.R.; Yang, H.C.; Pon, L.A. Mitochondrial inheritance in budding yeast. Traffic 2001, 2, 368–374. [Google Scholar]
- Mannella, C.A. Structure of the outer mitochondrial membrane: Ordered arrays of porelike subunits in outer-membrane fractions from Neurospora crassa mitochondria. J. Cell Biol 1982, 94, 680–687. [Google Scholar]
- Lindén, M.; Gellerfors, P. Hydrodynamic properties of porin isolated from outer membranes of rat liver mitochondria. Biochim. Biophys. Acta 1983, 736, 125–129. [Google Scholar]
- Shi, Y.; Jiang, C.; Chen, Q.; Tang, H. One-step on-column affinity refolding purification and functional analysis of recombinant human VDAC1. Biochem. Biophys. Res. Commun 2003, 303, 475–482. [Google Scholar]
- Shoshan-Barmatz, V.; Zalk, R.; Gincel, D.; Vardi, N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim. Biophys. Acta 2004, 1657, 105–114. [Google Scholar]
- Zalk, R.; Israelson, A.; Garty, E.S.; Azoulay-Zohar, H.; Shoshan-Barmatz, V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem. J 2005, 386, 73–83. [Google Scholar]
- Goncalves, R.P.; Buzhynskyy, N.; Prima, V.; Sturgis, J.N.; Scheuring, S. Supramolecular assembly of VDAC in native mitochondrial outer membranes. J. Mol. Biol 2007, 369, 413–418. [Google Scholar]
- Hoogenboom, B.W.; Suda, K.; Engel, A.; Fotiadis, D. The supramolecular assemblies of voltage-dependent anion channels in the native membrane. J. Mol. Biol 2007, 370, 246–255. [Google Scholar]
- Cowan, S.W.; Schirmer, T.; Rummel, G.; Steiert, M.; Ghosh, R.; Pauptit, R.A.; Jansonius, J.N.; Rosenbusch, J.P. Crystal structures explain functional properties of two E. coli porins. Nature 1992, 358, 727–733. [Google Scholar]
- de Pinto, V.; Benz, R.; Palmieri, F. Interaction of non-classical detergents with the mitochondrial porin. A new purification procedure and characterization of the pore-forming unit. Eur. J. Biochem 1989, 183, 179–187. [Google Scholar]
- Freitag, H.; Genchi, G.; Benz, R.; Palmieri, F; Neupert, W. Isolation of mitochondrial porin from Neurospora crassa. FEBS Lett. 1982, 145, 72–76. [Google Scholar]
- Freitag, H.; Neupert, W.; Benz, R. Purification and characterisation of a pore protein of the outer mitochondrial membrane from Neurospora crassa. Eur. J. Biochem 1982, 123, 629–636. [Google Scholar]
- le Saux, A.; Roux, P.; Trezeguet, V.; Fiore, C.; Schwimmer, C.; Dianoux, A.C.; Vignais, P.V.; Brandolin, G.; Lauquin, G.J. Conformational changes of the yeast mitochondrial adenosine diphosphate/adenosine triphosphate carrier studied through its intrinsic fluorescence. 1. Tryptophanyl residues of the carrier can be mutated without impairing protein activity. Biochemistry 1996, 35, 16116–16124. [Google Scholar]
- Popp, B.; Schmid, A.; Benz, R. Role of sterols in the functional reconstitution of water-soluble mitochondrial porins from different organisms. Biochemistry 1995, 34, 3352–3361. [Google Scholar]
- Kramer, R. Cholesterol as activator of ADP-ATP exchange in reconstituted liposomes and in mitochondria. Biochim. Biophys. Acta 1982, 693, 296–304. [Google Scholar]
- Rostovtseva, T.K.; Kazemi, N.; Weinrich, M.; Bezrukov, S.M. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem 2006, 281, 37496–37506. [Google Scholar]
- Jacobus, W.E.; Lehninger, A.L. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J. Biol. Chem 1973, 248, 4803–4810. [Google Scholar]
- Bessman, S.P.; Carpenter, C.L. The creatine-creatine phosphate energy shuttle. Annu. Rev. Biochem 1985, 54, 831–862. [Google Scholar]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The “phosphocreatine circuit” for cellular energy homeostasis. Biochem. J 1992, 281, 21–40. [Google Scholar]
- Schlattner, U.; Forstner, M.; Eder, M.; Stachowiak, O.; Fritz-Wolf, K.; Wallimann, T. Functional aspects of the X-ray structure of mitochondrial creatine kinase: A molecular physiology approach. Mol. Cell. Biochem 1998, 184, 125–140. [Google Scholar]
- Schlattner, U.; Tokarska-Schlattner, M.; Ramirez, S.; Bruckner, A.; Kay, L.; Polge, C.; Epand, R.F.; Lee, R.M.; Lacombe, M.L.; Epand, R.M. Mitochondrial kinases and their molecular interaction with cardiolipin. Biochim. Biophys. Acta 2009, 1788, 2032–2047. [Google Scholar]
- Hovius, R.; Lambrechts, H.; Nicolay, K.; de Kruijff, B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim. Biophys. Acta 1990, 29, 217–226. [Google Scholar]
- Schlattner, U.; Gehring, F.; Vernoux, N.; Tokarska-Schlattner, M.; Neumann, D.; Marcillat, O.; Vial, C.; Wallimann, T. C-terminal lysines determine phospholipid interaction of sarcomeric mitochondrial creatine kinase. J. Biol. Chem 2004, 279, 24334–24342. [Google Scholar]
- Hoppel, C.; Kerner, J.; Turkaly, P.; Minkler, P.; Tandler, B. Isolation of hepatic mitochondrial contact sites: Previously unrecognized inner membrane components. Anal. Biochem 2002, 302, 60–69. [Google Scholar]
- Speer, O.; Bäck, N.; Buerklen, T.; Brdiczka, D.; Koretsky, A.; Wallimann, T.; Eriksson, O. Octameric mitochondrial creatine kinase induces and stabilizes contact sites between the inner and outer membrane. Biochem. J 2005, 385, 445–450. [Google Scholar]
- Brdiczka, D.G.; Zorov, D.B.; Sheu, S.S. Mitochondrial contact sites: Their role in energy metabolism and apoptosis. Biochim. Biophys. Acta 2006, 1762, 148–163. [Google Scholar]
- Claypool, S.M.; Oktay, Y.; Boontheung, P.; Loo, J.A.; Koehler, C.M. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol 2008, 182, 937–950. [Google Scholar]
- Brandolin, G.; le Saux, A.; Trézéguet, V.; Vignais, P.V.; Lauquin, G.J.-M. Biochemical characterisation of the isolated Anc2 adenine nucleotide carrier from Saccharomyces cerevisiae Mitochondria. Biochem. Biophys. Res. Commun 1993, 192, 143–150. [Google Scholar]
- Hunte, C.; Richers, S. Lipids and membrane protein structures. Curr. Opin. Struct. Biol 2008, 406–411. [Google Scholar]
- Booth, P.J.; Curran, A.R. Membrane protein folding. Curr. Opin. Struct. Biol 1999, 9, 115–195. [Google Scholar]
- Dowhan, W.; Bogdanov, M. Lipid-dependent membrane protein topogenesis. Annu. Rev. Biochem 2009, 78, 515–540. [Google Scholar]
- Dienhart, M.K.; Rosemary, A.M. The yeast Aac2 protein exists in physical association with the cytochrome bc1-COX supercomplex and the TIM23 machinery. Mol. Biol. Cell 2008, 19, 3934–3943. [Google Scholar]
- Harner, M.; Körner, C.; Walther, D.; Mokranjac, D.; Kaesmacher, J.; Welsch, U.; Griffith, J.; Mann, U.; Reggiori, F.; Neupert, W. The mitochondrial contact site complex a determinant of mitochondrial architecture. EMBO J 2011, 30, 4356–4370. [Google Scholar]
- Hoppins, S.; Collins, S.R.; Cassidy-Stone, A.; Hummel, E.; DeVay, R.M.; Lackner, L.L.; Westermann, B.; Schuldiner, M.; Weissman, J.S.; Nunnari, J. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J. Cell Biol 2011, 195, 323–340. [Google Scholar]
- von der Malsburg, K.; Müller, J.M.; Bohnert, M.; Oeljeklaus, S.; Kwiatkowska, P.; Becker, T.; Loniewska-Lwowska, A.; Wiese, S.; Rao, S.; Milenkovic, D.; et al. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev. Cell 2011, 21, 694–707. [Google Scholar]
- Wittig, I.; Schägger, H. Structural organization of mitochondrial ATP synthase. Biochim. Biophys. Acta 2008, 1777, 592–598. [Google Scholar]
- Nunnari, J.; Marshall, W.F.; Straight, A.; Murray, A.; Sedat, J.W.; Walter, P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol. Biol. Cell 1997, 8, 1233–1242. [Google Scholar]




| Protein partners | Interaction partners domain | Mediators | Interaction VDAC * domain | Roles | Protein partners | References | ||
|---|---|---|---|---|---|---|---|---|
| activators (+) | inhibitors (−) | |||||||
| Hexokinase I and II (HK I and HK II) | N-terminal hydrophobic α-helical (MIASHLLAYFFTELM) | Mg2+, Residues E188 and E202 of VDAC | Glucose-6-phosphate (G6P) and DCCD * | Amino acids | Location in the structure | PTP * | Mitochondrial apoptosis | |
| E72 | β sheet n°4 | [140–142] | ||||||
| E65 | Cytosolic loop n°2 | [143] | ||||||
| D77 | Matrix loop n°2 | |||||||
| K73 | β sheet n°4 | |||||||
| Aacp * | Cardiolipin-induced | [144–146] | ||||||
| MtCK * | Cardiolipin-induced | [147–150] | ||||||
| Bcl2 family proteins | H5 and H6 transmembrane helix | - | - | PTP | Mitochondrial apoptosis | [151–154] | ||
| Tubulin | Tubulin anionic C-terminal tail (CTT) peptides | - | VDAC lumen | regulation | Mitochondrial respiration | [155–158] | ||
| Inositol 1,4,5- triphosphate receptor (IP3R) | N-terminal domain | Chaperone grp75 | - | Scaffolding the ER *-mitochondria contacts | Ca2+ uptake into mitochondria | [159] | ||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Clémençon, B. Yeast Mitochondrial Interactosome Model: Metabolon Membrane Proteins Complex Involved in the Channeling of ADP/ATP. Int. J. Mol. Sci. 2012, 13, 1858-1885. https://doi.org/10.3390/ijms13021858
Clémençon B. Yeast Mitochondrial Interactosome Model: Metabolon Membrane Proteins Complex Involved in the Channeling of ADP/ATP. International Journal of Molecular Sciences. 2012; 13(2):1858-1885. https://doi.org/10.3390/ijms13021858
Chicago/Turabian StyleClémençon, Benjamin. 2012. "Yeast Mitochondrial Interactosome Model: Metabolon Membrane Proteins Complex Involved in the Channeling of ADP/ATP" International Journal of Molecular Sciences 13, no. 2: 1858-1885. https://doi.org/10.3390/ijms13021858