Presence of CP4-EPSPS Component in Roundup Ready Soybean-Derived Food Products
Abstract
:1. Introduction
2. Results and Discussion
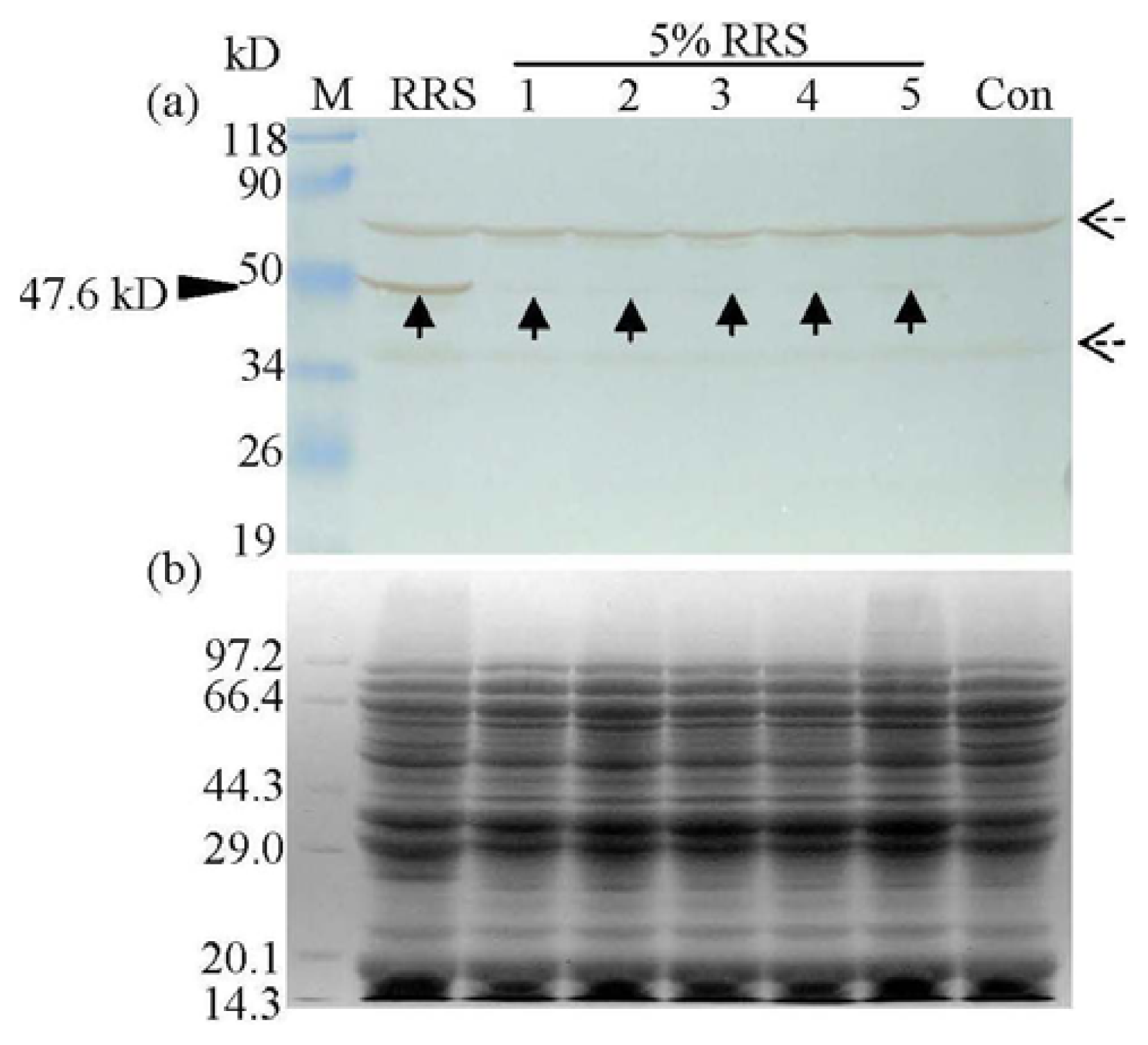
2.1. Detection of Transgenic Components in Commercial Soya Protein Concentrates
2.2. Effect of Single-Factor Treatment on CP4-EPSPS Protein Degradation
2.3. Effect of Food Matrix on CP4-EPSPS Protein Degradation and Traceability during Food Processing
3. Experimental Section
3.1. Plant Materials
3.2. Heat Processing Operation
3.3. Microwave Treatment
3.4. Autoclaving
3.5. Food Matrix
3.6. DNA Extraction
3.7. PCR Reaction
3.8. Protein Extraction
3.9. Western Blotting Analysis
4. Conclusions
Acknowledgements
References
- Azadi, H.; Ho, P. Genetically modified and organic crops in developing countries: A review of options for food security. Biotechnol. Adv 2010, 28, 160–168. [Google Scholar]
- Xu, Z.H.; Bai, S.N. Impact of biotechnology on agriculture in China. Trends Plant Sci 2002, 7, 374–375. [Google Scholar]
- Hammond, B.G.; Jez, J.M. Impact of food processing on the safety assessment for proteins introduced into biotechnology-derived soybean and corn crops. Food Chem. Toxicol 2011, 49, 711–721. [Google Scholar]
- Moriuchi, R.; Monma, K.; Sagi, N.; Uno, N.; Kamata, K. Applicability of quantitative PCR to soy processed foods containing Roundup Ready Soy. Food Control 2007, 8, 191–195. [Google Scholar]
- Brod, F.C.A.; Ferrari, C.D.S.; Valente, L.L.V.; Arisi, A.C.M. Nested PCR detection of genetically modified soybean in soybean flour, infant formula and soymilk. Food Sci. Technol 2007, 40, 748–751. [Google Scholar]
- Costa, J.; Mafra, I.; Amaral, J.S.; Olivreira, M.B.P.P. Monitoring genetically modified soybean along the industrial soybean oil extraction and refining processes by polymerase chain reaction techniques. Food Res. Int 2010, 43, 301–306. [Google Scholar]
- Uzogara, S.G. The impact of genetic modification of human foods in the 21st century: A review. Biotechnol. Adv 2000, 18, 179–206. [Google Scholar]
- Tripathi, L. Techniques for detecting genetically modified crops and products. Afr. J. Biotechnol 2005, 4, 1472–1479. [Google Scholar]
- Anklam, E.; Gadani, F.; Heinze, P.; Pijnenburg, H.; van den Eede, G. Analytical methods for detection and determination of genetically modified organisms in agricultural crops and plant-derived food products. Eur. Food Res. Technol 2002, 214, 3–26. [Google Scholar]
- Ahmed, F.E. Detection of genetically modified organisms in foods. Trends Biotechnol 2002, 20, 215–223. [Google Scholar]
- Rogan, G.J.; Dubin, Y.A.; Lee, T.C.; Magin, K.M.; Astwood, J.D.; Bhakta, N.S.; Leach, J.N.; Sanders, P.R.; Fuchs, R.L. Immunodiagnostic methods for detection of 5-enolpyruvylshikimate-3-phosphate synthase in Roundup Ready® soybeans. Food Control 1999, 10, 407–414. [Google Scholar]
- Lipp, M.; Brodmann, P.; Pietsch, K.; Pauwels, J.; Anklam, E. IUPAC collaborative trial study of a method to detect genetically modified soy beans and maize in dried powder. J. AOAC Int 1999, 82, 923–928. [Google Scholar]
- Gasser, C.S.; Winter, J.A.; Hironaka, C.M.; Shah, D.M. Structure, expression, and evolution of the 5-enolpyruvylshikimate-3-phosphate synthase genes of petunia and tomato. J. Biol. Chem 1988, 263, 4280–4287. [Google Scholar]
- van Duijn, G.; van Biert, R.; Bleeker-Marcelis, H.; Peppelman, H.; Hessing, M. Detection methods for genetically modified crops. Food Control 1999, 10, 375–378. [Google Scholar]
- Chen, Y.; Wang, Y.; Ge, Y.; Xu, B. Degradation of endogenous and exogenous genes of Roundup-Ready soybean during food processing. J. Agric. Food Chem 2005, 53, 10239–10243. [Google Scholar]
- Corbisier, P.; Trapmann, S.; Gancberg, D.; Hannes, L.; Van Iwaarden, P.; Berben, G.; Schimmel, H.; Emons, H. Quantitative determination of Roundup Ready soybean (Glycine max) extracted from highly processed flour. Anal. Bioanal. Chem 2005, 383, 282–290. [Google Scholar]
- Liu, Y.C.; Lin, S.H.; Lai, H.M.; Jeng, S.T. Detection of genetically modified soybean and its product tou-kan by polymerase chain reaction with dual pairs of DNA primers. Eur. Food Res. Technol 2005, 221, 725–730. [Google Scholar]
- Vijayakumar, K.R.; Martin, A.; Gowda, L.R.; Prakash, V. Detection of genetically modified soya and maize: Impact of heat processing. Food Chem 2009, 117, 514–521. [Google Scholar]
- Rousu, M.; Huffman, W.E.; Shogren, J.F.; Tegene, A. Are United States consumers tolerant of genetically modified foods? Rev. Agric. Econ 2003, 26, 19–31. [Google Scholar]
- Ogasawara, T.; Arakawa, F.; Akiyama, H.; Goda, Y.; Ozeki, Y. Fragmentation of DNAs of processed foods made from genetically modified soybeans. Jpn. J. Food Chem 2003, 10, 155–160. [Google Scholar]
- Chen, Y.; Ge, Y.Q.; Wang, Y. Effect of critical processing procedures on transgenic components in quality and quantity level during soymilk processing of Roundup Ready Soybean. Eur. Food Res. Technol 2007, 225, 119–126. [Google Scholar]
- Gryson, N. Effect of food processing on plant DNA degradation and PCR-based GMO analysis: A review. Anal. Bioanal. Chem 2010, 396, 2003–2022. [Google Scholar]






| Gene | Location | Primer sequence | Amplicon size (bp) | Annealing temperature |
|---|---|---|---|---|
| cp4-epsps (AB209952.1) | 298–1881 | 5′-ATGGCACAAATTAACAACAT-3′ 5′-TCAGGCAGCCTTCGTATC-3′ | 1584 | 52 |
| 517–854 | 5′-CTTCACGGTGCAAGCAGC-3′ 5′-TCGTAGACCCCGACGAGG-3′ | 338 | 50 | |
| 614–1111 | 5′-CCTTCATGTTCGGCGGTCTCG-3′ 5′-ACGTCATGATCGGCTCGATG-3′ | 498 | 55 | |
| 843–1123 | 5′-CGGGGTCTACGATTTCGA-3′ 5′-GCCCTGCAGCATCTTTTC-3′ | 281 | 56 | |
| 1111–1429 | 5′-TGCGATCATACGGAAAAG-3′ 5′-CAGCGTGGAGGAGCGAAC-3′ | 319 | 52 | |
| 1431–1780 | 5′-TCGCTCCTCCACGCTGAA-3′ 5′-CCGTGACAGGGTTTTCCG-3′ | 350 | 50 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, H.; Zhang, Y.; Zhu, C.; Xiao, X.; Zhou, X.; Xu, S.; Shen, W.; Huang, M. Presence of CP4-EPSPS Component in Roundup Ready Soybean-Derived Food Products. Int. J. Mol. Sci. 2012, 13, 1919-1932. https://doi.org/10.3390/ijms13021919
Wu H, Zhang Y, Zhu C, Xiao X, Zhou X, Xu S, Shen W, Huang M. Presence of CP4-EPSPS Component in Roundup Ready Soybean-Derived Food Products. International Journal of Molecular Sciences. 2012; 13(2):1919-1932. https://doi.org/10.3390/ijms13021919
Chicago/Turabian StyleWu, Honghong, Yu Zhang, Changqing Zhu, Xiao Xiao, Xinghu Zhou, Sheng Xu, Wenbiao Shen, and Ming Huang. 2012. "Presence of CP4-EPSPS Component in Roundup Ready Soybean-Derived Food Products" International Journal of Molecular Sciences 13, no. 2: 1919-1932. https://doi.org/10.3390/ijms13021919





