An Oligodeoxynucleotide That Induces Differentiation of Bone Marrow Mesenchymal Stem Cells to Osteoblasts in Vitro and Reduces Alveolar Bone Loss in Rats with Periodontitis
Abstract
:1. Introduction
2. Results and Discussion
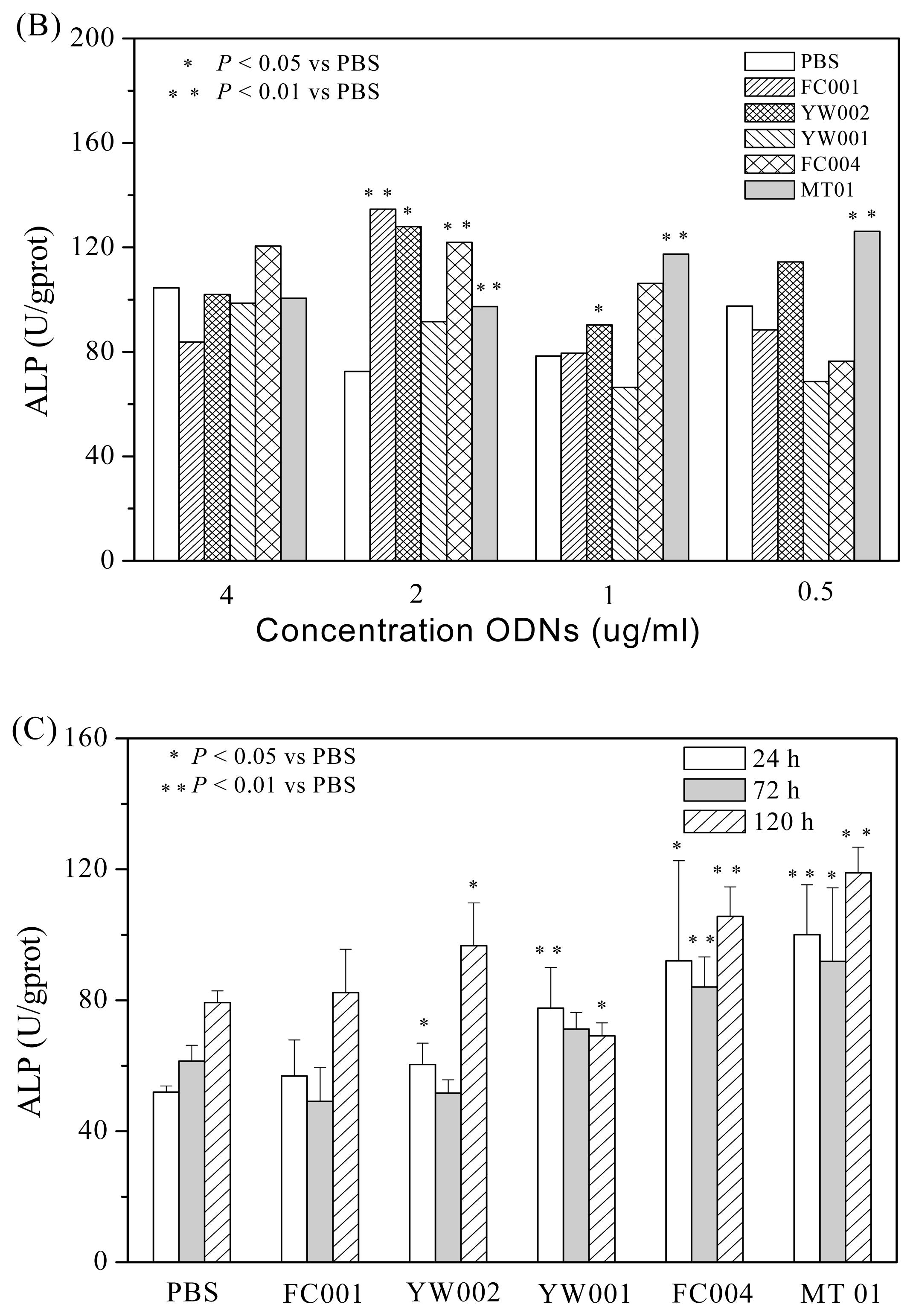
2.1. Screening of ODNs Capable of Stimulating the Proliferation and Differentiation of Rat BMSCs
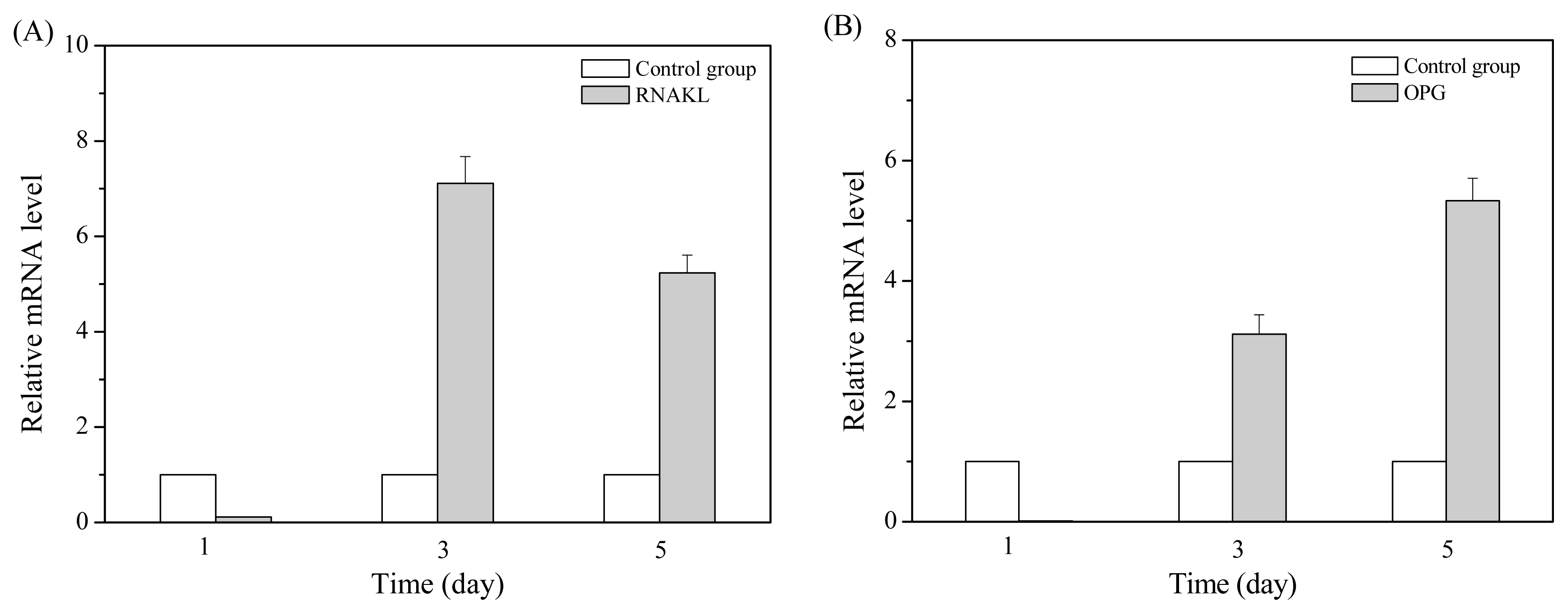
2.2. MT01 Induces the Differentiation of BMSCs to Osteoblasts and Its Possible Mechanism
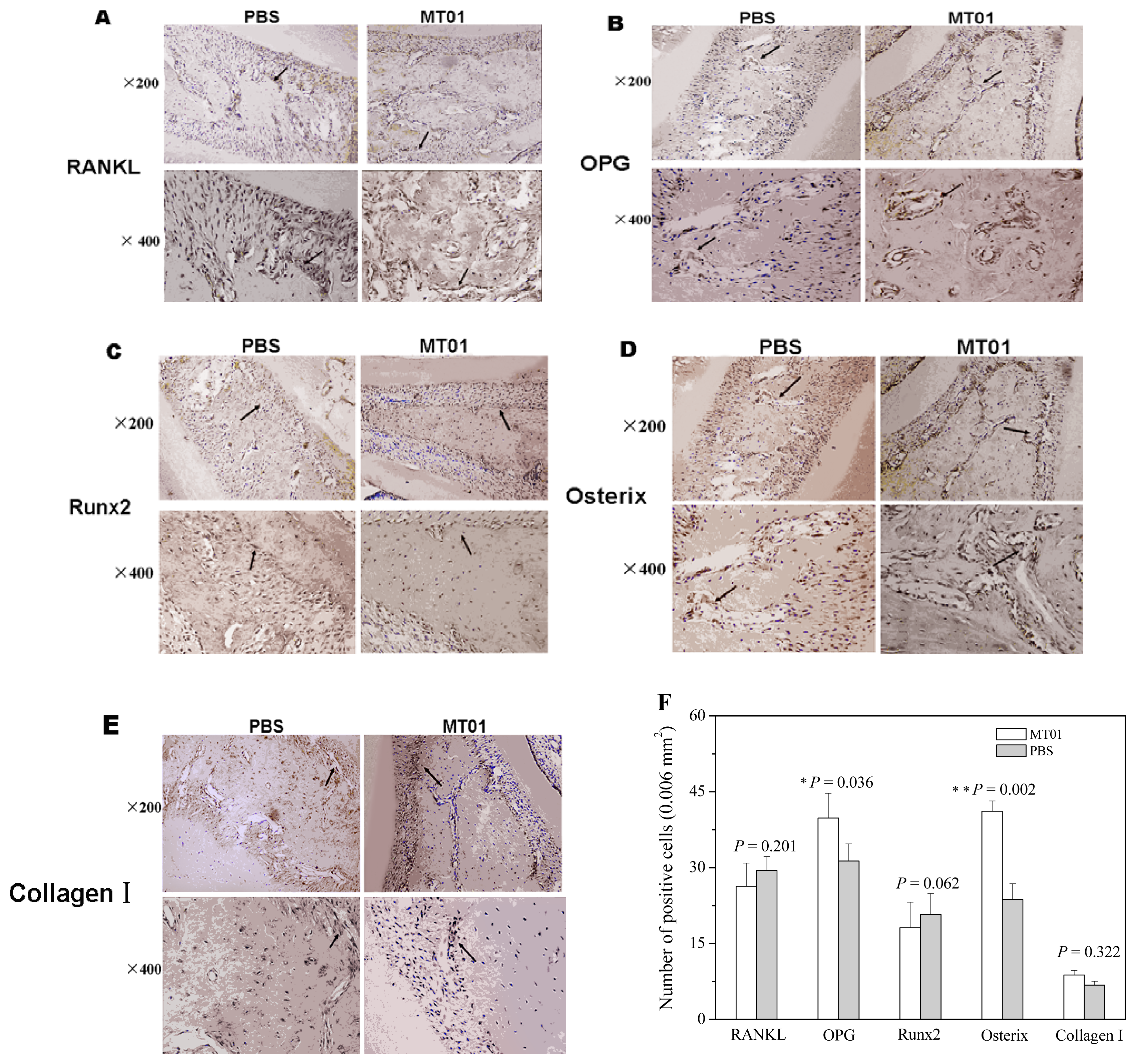
2.3. MT01 Mediated Reduction of Alveolar Bone Loss in Experimental Periodontitis Rats
3. Experimental Section
3.1. Materials
3.2. Isolation, Culture and Identification of BMSCs
3.3. Proliferation Assay
3.4. ALP Analysis in BMSCs
3.5. Quantitative Real-Time PCR
3.6. Rat Experiment
3.7. Microscopic Examination of Alveolar Bone Loss
3.8. Immunohistochemistry of RANKL, OPG, Osterix, Runx2 and Collagen I
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Lin, N.H.; Gronthos, S.; Mark, B.P. Stem cells and future periodontal regeneration. Periodontology 2009, 51, 239–251. [Google Scholar]
- He, H.; Cao, J.; Wang, D.; Gu, B.; Guo, H.; Liu, H. Gene-modified stem cells combined with rapid prototyping techniques: A novel strategy for periodontal regeneration. Stem Cell Rev 2010, 6, 137–141. [Google Scholar]
- Mudda, J.A.; Bajaj, M. Stem cell therapy: A challenge to periodontist. Indian J. Dent. Res 2011, 22, 132–139. [Google Scholar]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res 2009, 88, 792–806. [Google Scholar]
- Johnson, B.Q.; Fox, R.; Chen, X.; Thibeault, S. Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injections in rats. Laryngoscope 2010, 120, 537–545. [Google Scholar]
- Kobayashi, Y.; Udagawa, N. Mechanisms of alveolar bone remodeling. Clin. Calcium 2007, 17, 209–216. [Google Scholar]
- Morsczeck, C.; Schmalz, G.; Reichert, T.E.; Völlner, F.; Galler, K.; Driemel, O. Somatic stem cells for regenerative dentistry. Clin. Oral. Investig 2008, 12, 113–118. [Google Scholar]
- Oz, H.S.; Puleo, D.A. Animal models for periodontal disease. J. Biomed. Biotechnol 2011, 2011, 1–8. [Google Scholar]
- Nakajima, K.; Hamada, N.; Takahashi, Y.; Sasaguri, K.; Tsukinoki, K.; Umemoto, T.; Sato, S. Restraint stress enhances alveolar bone loss in an experimental rat model. J. Periodontal. Res 2006, 41, 527–534. [Google Scholar]
- Klausen, B.; Sfintescu, C.; Evans, R.T. Asymmetry in periodontal bone loss of gnotobiotic Sprague-Dawley rats. Arch. Oral Biol 1991, 36, 685–687. [Google Scholar]
- Jönsson, D.; Nebel, D.; Bratthall, G.; Nilsson, B.O. The human periodontal ligament cell: A fibroblast-like cell acting as an immune cell. J. Periodontal Res 2011, 46, 153–157. [Google Scholar]
- Schwartz, Z.; Goultschin, J.; Dean, D.D.; Boyan, B.D. Mechanisms of alveolar bone destruction in periodontitis. Periodontology 1997, 14, 158–172. [Google Scholar]
- Wiebe, S.H.; Hafezi, M.; Sandhu, H.S.; Sims, S.M.; Dixon, S.J. Osteoclast activation in inflammatory periodontal diseases. Oral Dis 1996, 2, 167–180. [Google Scholar]
- Harada, S.; Takahashi, N. Control of bone resorption by RANKL-RANK system. Clin. Calcium 2011, 21, 1121–1130. [Google Scholar]
- Han, X.; Kawai, T. Immune response: The key to bone resorption in periodontal disease. J. Periodontol 2005, 76, 2033–2041. [Google Scholar]
- Cochran, D.L. Inflammation and bone loss in periodontal disease. J. Periodontol 2008, 79, 1569–1576. [Google Scholar]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys 2008, 15, 139–146. [Google Scholar]
- Komori, T. Regulation of bone development and maintenance by Runx2. Front. Biosci 2008, 13, 898–903. [Google Scholar]
- Zhu, J.; Shimizu, E.; Zhang, X.; Partridge, N.C.; Qin, L. EGFR signaling suppresses osteoblast differentiation and inhibits expression of master osteoblastic transcription factors Runx2 and Osterix. J. Cell. Biochem 2011, 112, 1749–1760. [Google Scholar]
- Wada, S.; Kamiya, S. Bone and bone related biochemical examinations. Bone and collagen related metabolites. Clin. Calcium 2006, 16, 1017–1021. [Google Scholar]
- Darby, I. Periodontal materials. Aust. Dent. J 2011, 56, 107–118. [Google Scholar]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials—Biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol 2008, 35, 106–116. [Google Scholar]
- Zou, W.; Schwartz, H.; Endres, S.; Hartmann, G.; Bar-Shavit, Z. CpG oligonucleotides: Novel regulators of osteoclast differentiation. FASEB J 2002, 16, 274–282. [Google Scholar]
- Amcheslavsky, A.; Zou, W.; Bar-Shavit, Z. Toll-like receptor 9 regulates tumor necrosis factor-α expression by different mechanisms implications for osteoclastogenesis. J. Biol. Chem 2004, 279, 54039–54045. [Google Scholar]
- Chang, J.H.; Chang, E.J.; Kim, H.H.; Kim, S.K. Enhanced inhibitory effects of a novel CpG motif on osteoclast differentiation via TREM-2 down-regulation. Biochem. Biophys. Res. Commun 2009, 389, 28–33. [Google Scholar]
- Amcheslavsky, A.; Hemmi, H.; Akira, S.; Bar-Shavit, Z. Differential contribution of osteoclast- and osteoblast-lineage cells to CpG-oligodeoxynucleotide (CpG-ODN) modulation of osteoclastogenesis. J. Bone Miner. Res 2005, 20, 1692–1699. [Google Scholar]
- Zou, W.; Amcheslavsky, A.; Bar-Shavit, Z. CpG Oligodeoxynucleotides modulate the osteoclastogenic activity of osteoblasts via toll-like receptor 9. J. Biol. Chem 2003, 278, 16732–16740. [Google Scholar]
- Amcheslavsky, A.; Bar-Shavit, Z. Interleukin (IL)-12 mediates the anti-osteoclastogenic activity of CpG-oligodeoxynucleotides. J. Cell. Physiol 2006, 207, 244–250. [Google Scholar]
- Yang, G.; Wan, M.; Zhang, Y.S.; Sun, L.G.; Sun, R.; Hu, D.L.; Zhou, X.J.; Wang, L.; Wu, X.L.; Wang, L.Y.; et al. Inhibition of a C-rich oligodeoxynucleotide on activation of immune cells in vitro and enhancement of antibody response in mice. Immunology 2010, 131, 501–512. [Google Scholar]
- Feng, Z.; Shen, Y.; Wang, L.; Cheng, L.; Wang, J.; Li, Q.; Shi, W.; Sun, X. An oligodeoxynucleotide with promising modulation activity for the proliferation and activation of osteoblast. Int. J. Mol. Sci 2011, 12, 2543–2555. [Google Scholar]
- Sun, R.; Sun, L.G.; Bao, M.S.; Zhang, Y.S.; Wang, L.; Wu, X.L.; Hu, D.L.; Liu, Y.J.; Yu, Y.L.; Wang, L.Y. A human microsatellite DNA-mimicking oligodeoxynucleotide with CCT repeats negatively regulates TLR7/9-mediated innate immune responses via selected TLR pathways. Clin. Immunol 2010, 134, 262–276. [Google Scholar]
- Bao, M.; Zhang, Y.; Wan, M.; Dai, L.; Hu, X.P.; Wu, X.L.; Wang, L.; Deng, P.; Wang, J.Z.; Chen, J.Z. Anti-SARS-CoV immunity induced by a novel CpG oligodeoxynucleotide. Clin. Immunol 2006, 118, 180–187. [Google Scholar]
- Wang, X.; Bao, M.; Wan, M.; Wei, H.F.; Wang, L.; Yu, H.T.; Zhang, X.S.; Yu, Y.L.; Wang, L.Y. A CpG oligodeoxynucleotide acts as a potent adjuvant for inactivated rabies virus vaccine. Vaccine 2008, 26, 1893–1901. [Google Scholar]
- Yang, L.; Sun, L.G.; Wu, X.L.; Wang, L.; Wei, H.F.; Wan, M.; Zhang, P.Y.; Yu, Y.L.; Wang, L.Y. Therapeutic injection of C-class CpG ODN in draining lymph node area induces potent activation of immune cells and rejection of established breast cancer in mice. Clin. Immunol 2009, 131, 426–437. [Google Scholar]
- Jacobson, A.; Johansson, S.; Branting, M.; Melhus, H. Vitamin A differentially regulates RANKL and OPG expression in human osteoblasts. Biochem. Biophys. Res. Commun 2004, 322, 162–167. [Google Scholar]
- Pratap, J.; Galindo, M.; Zaidi, S.K.; Vradii, D.; Bhat, B.M.; Robinson, J.A.; Choi, J.-Y.; Komori, T.; Stein, J.L.; et al. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res 2003, 63, 5357–5362. [Google Scholar]
- Kim, Y.-J.; Kim, H.-N.; Park, E.-K.; Lee, B.-H.; Ryoo, H.-M.; Kim, S.-Y.; Kim, I.-S.; Stein, J.L.; Lian, J.B.; Stein, G.S. The bone-related Zn finger transcription factor Osterix promotes proliferation of mesenchymal cells. Gene 2006, 366, 145–151. [Google Scholar]
- Cai, X.; Li, C.; Du, G.; Cao, Z. Protective effects of baicalin on ligature induced periodontitis in rats. J. Periodontal Res 2008, 43, 14–21. [Google Scholar]
- SPOT RT Software, version 3.5; Spot Diagnostic Instruments: Sterling Heights, MI, USA, 2001.
- Ning, J.; Li, C.; Li, H.; Chang, J. Bone marrow mesenchymal stem cells differentiate into urothelial cells and the implications for reconstructing urinary bladder mucosa. Cytotechnology 2011, 63, 531–539. [Google Scholar]
- Guida, L.; Annunziata, M.; Passaro, I.; Buonaiuto, C.; Rullo, R.; Tetè, S.; Ragione, D.F.; Oliva, A. Acetylsalicylic acid inhibits proliferation of human bone marrow stromal cells and matrix mineralization. Int. J. Immunopathol. Pharmacol 2008, 21, 921–928. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc 2008, 3, 1101–1108. [Google Scholar]
- Schou, S.; Holmstrup, P.; Stoltze, K.; Hjørting-Hansen, E.; Kornman, K.S. Ligature-induced marginal inflammation around osseointegrated implants and ankylosed teeth. Clin. Oral Implants Res 1993, 4, 12–22. [Google Scholar]




© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shen, Y.; Feng, Z.; Lin, C.; Hou, X.; Wang, X.; Wang, J.; Yu, Y.; Wang, L.; Sun, X. An Oligodeoxynucleotide That Induces Differentiation of Bone Marrow Mesenchymal Stem Cells to Osteoblasts in Vitro and Reduces Alveolar Bone Loss in Rats with Periodontitis. Int. J. Mol. Sci. 2012, 13, 2877-2892. https://doi.org/10.3390/ijms13032877
Shen Y, Feng Z, Lin C, Hou X, Wang X, Wang J, Yu Y, Wang L, Sun X. An Oligodeoxynucleotide That Induces Differentiation of Bone Marrow Mesenchymal Stem Cells to Osteoblasts in Vitro and Reduces Alveolar Bone Loss in Rats with Periodontitis. International Journal of Molecular Sciences. 2012; 13(3):2877-2892. https://doi.org/10.3390/ijms13032877
Chicago/Turabian StyleShen, Yuqin, Zhiyuan Feng, Chongtao Lin, Xu Hou, Xueju Wang, Jing Wang, Yongli Yu, Liying Wang, and Xinhua Sun. 2012. "An Oligodeoxynucleotide That Induces Differentiation of Bone Marrow Mesenchymal Stem Cells to Osteoblasts in Vitro and Reduces Alveolar Bone Loss in Rats with Periodontitis" International Journal of Molecular Sciences 13, no. 3: 2877-2892. https://doi.org/10.3390/ijms13032877



