Cellulose Biosynthesis Inhibitors: Comparative Effect on Bean Cell Cultures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of Callus Growth
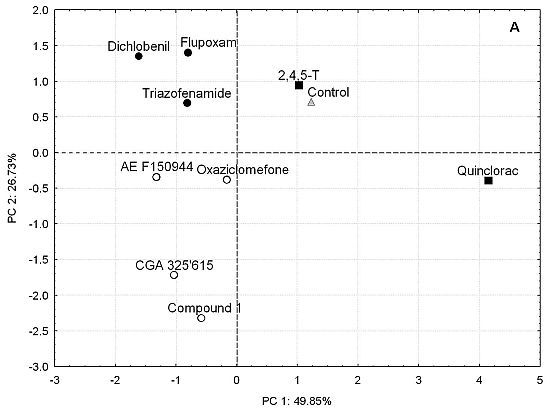
2.2. Uptake of [14C]Glucose after Short-Term Exposure to the Inhibitors
3. Experimental Section
3.1. Cell Cultures
3.2. Effect of Inhibitors on Calluses Growth
3.3. Cellulose Analysis
3.4. [14C]Glucose Uptake
3.5. Cell Wall Fractionation
4. Conclusions
Acknowledgments
References
- Doblin, M.S.; Pettolino, F.; Bacic, A. Plant cell walls: the skeleton of the plant world. Funct. Plant Physiol 2010, 37, 357–381. [Google Scholar]
- Ringli, C. Monitoring the outside: Cell wall-sensing mechanisms. Plant Physiol 2010, 153, 1445–1452. [Google Scholar]
- Guerreiro, G.; Fugelstad, J.; Bulone, V. What do really know about cellulose biosynthesis in higher plants? J. Integra. Plant Biol 2010, 52, 161–175. [Google Scholar]
- Acebes, J.L.; Encina, A.; García-Angulo, P.; Alonso-Simón, A.; Mélida, H.; Álvarez, J.M. Cellulose Biosynthesis Inhibitors: Their Uses as Potential Herbicides and as Tools in Cellulose and Cell Wall Structural Plasticity Research. In Cellulose: Structure and Properties, Derivatives and Industrial Uses; Lejeune, A., Deprez, T., Eds.; Nova Publishers: New York, NY, USA, 2010; pp. 39–73. [Google Scholar]
- Delmer, D.P.; Read, S.M.; Cooper, G. Identification of a receptor protein in cotton fibers for the herbicide 2,6-dichlorobenzonitrile. Plant Physiol 1987, 84, 415–420. [Google Scholar]
- Kiedaisch, B.M.; Blanton, R.L.; Haigler, C.H. Characterization of a novel cellulose synthesis inhibitor. Planta 2003, 217, 922–30. [Google Scholar]
- Hoffman, J.C.; Vaughn, K.C. Flupoxam induces classic club root morphology but is not a mitotic disrupter herbicide. Pestic. Biochem. Physiol 1996, 55, 49–53. [Google Scholar]
- Heim, D.R.; Larrinua, I.M.; Murdoch, M.G.; Roberts, J.L. Triazofenamide is a cellulose biosynthesis inhibitor. Pestic. Biochem. Physiol 1998, 59, 163–168. [Google Scholar]
- Sharples, K.R.; Hawkes, T.R.; Mitchell, G.; Edwards, L.S.; Langford, M.P.; Langton, D.W.; Rogers, K.M.; Townson, J.K.; Wang, Y. A novel thiazolidinone herbicide is a potent inhibitor of glucose incorporation into cell wall material. Pestic. Sci 1998, 54, 368–376. [Google Scholar]
- Peng, L.; Xiang, F.; Roberts, E.; Kawagoe, Y.; Greve, L.C.; Kreuz, K.; Delmer, D.P. The experimental herbicide CGA 325′615 inhibits synthesis of crystalline cellulose and causes accumulation of non-crystalline β-(1,4)-glucan associated with CesA protein. Plant Physiol 2001, 126, 981–992. [Google Scholar]
- O’Looney, N.; Fry, S.C. The novel herbicide oxaziclomefone inhibits cell expansion in maize cell cultures without affecting turgor pressure or wall acidification. New Phytol 2005, 168, 1–7. [Google Scholar]
- O’Looney, N.; Fry, S.C. Oxaziclomefone, a new herbicide, inhibits wall expansion in maize cell-cultures without affecting polysaccharide biosynthesis, xyloglucan transglycosylation, peroxidase action or apoplastic ascorbate oxidation. Ann. Bot 2005, 96, 1–11. [Google Scholar]
- Koo, S.J.; Neal, J.C.; DiTomaso, J.M. Mechanism of action and selectivity of quinclorac in grass roots. Pestic. Biochem. Physiol 1997, 57, 44–53. [Google Scholar]
- Tresch, S.; Grossmann, K. Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots. Pestic. Biochem. Physiol 2003, 75, 73–78. [Google Scholar]
- Peng, L.; Kawagoe, Y.; Hogan, P.; Delmer, D. Sitosterol-β-Glucoside as primer for cellulose synthesis in plants. Science 2002, 295, 147–150. [Google Scholar]
- DeBolt, S.; Gutierrez, R.; Ehrhardt, D.W.; Somerville, C. Nonmotile cellulose synthase subunits repeatedly accumulate within localized regions at the plasma membrane in Arabidopsis hypocotyl cells following 2,6-dichlorobenzonitrile treatment. Plant Physiol 2007, 145, 334–338. [Google Scholar]
- Wightman, R.; Turner, S. Trafficking of the plant cellulose synthase complex. Plant Physiol 2010, 153, 427–432. [Google Scholar]
- Himmelspach, R.; Willamson, R.E.; Wasteneys, G.O. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J 2003, 36, 565–575. [Google Scholar]
- Nakagawa, N.; Sakurai, N. Increase in the amount of celA1 protein in tobacco BY-2 cells by a cellulose biosynthesis inhibitor, 2,6-dichlorobenzonitrile. Plant Cell Physiol 1998, 39, 779–785. [Google Scholar]
- Mélida, H.; Encina, A.; Álvarez, J.; Acebes, J.L.; Caparrós-Ruíz, D. Unraveling the biochemical and molecular networks involved in maize cell habituation to the cellulose biosynthesis inhibitor dichlobenil. Mol. Plant 2010, 3, 842–853. [Google Scholar]
- Rajangam, A.S.; Kumar, M.; Aspeborg, H.; Guerriero, G.; Arvestad, L.; Pansri, P.; Brown, C.J.L.; Hober, S.; Blomqvist, K.; Divne, C.; et al. MAP20, a microtubule-associated protein in the secondary cell walls of hybrid aspen, is a target of the cellulose synthesis inhibitor, 2,6-dichlorobenzonitrile. Plant Physiol 2008, 148, 1283–1294. [Google Scholar]
- Vaughn, K.C.; Turley, R.B. Ultrastructural effects of cellulose biosynthesis inhibitor herbicides on developing cotton fibers. Protoplasma 2001, 216, 80–93. [Google Scholar]
- Kurek, I.; Kawagoe, Y.; Jacob-Wilk, D.; Doblin, M.; Delmer, D. Dimerization of cotton fiber cellulose synthase catalytic subunits occurs via oxidation of the zinc-binding domains. Proc. Natl. Acad. Sci. USA 2002, 17, 11109–11114. [Google Scholar]
- Crowell, E.F.; Bischoff, V.; Desprez, T.; Rolland, A.; Stierhof, Y.; Schumacher, K.; Gonneau, M.; Höfte, H.; Vernhettes, S. Pausing of golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 2009, 21, 1141–1154. [Google Scholar]
- Alonso-Simón, A.; García-Angulo, P.; Encina, A.; Acebes, J.L.; Álvarez, J. Habituation of bean (Phaseolus vulgaris) cell cultures to quinclorac and analysis of the subsequent cell wall modifications. Ann. Bot 2008, 101, 1329–1339. [Google Scholar]
- Hofmannová, J.; Schwarzerová, K.; Havelková, L.; Boriková, P.; Petrásek, J.; Opatrny, Z. A novel, cellulose synthesis inhibitory action of ancymidol impairs plant cell expansion. J. Exp. Bot 2008, 59, 3963–3974. [Google Scholar]
- DeBolt, S.; Gutiérrez, R.; Ehrhardt, D.W.; Melo, C.V.; Ross, L.; Cutler, S.R.; Somerville, C.; Bonetta, D. Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proc. Natl. Acad. Sci. USA 2007, 104, 5854–5859. [Google Scholar]
- Yoneda, A.; Higaki, T.; Kutsuna, N.; Kondo, Y.; Osada, H.; Hasezawa, S.; Matsui, M. Chemical genetic screening identifies a novel inhibitor of parallel alignment of cortical microtubules and cellulose microfibrils. Plant Cell Physiol 2007, 48, 1393–1403. [Google Scholar]
- Yoneda, A.; Ito, T.; Higaki, T.; Kutsuna, N.; Saito, T.; Ishimizu, T.; Osada, H.; Hasezawa, S.; Matsui, M.; Demura, T. Cobtorin target analysis reveals that pectin functions in the deposition of cellulose microfibrils in parallel with cortical microtubules. Plant J 2010, 64, 657–667. [Google Scholar]
- Grossmann, K.; Tresch, S.; Plath, P. Triaziflam and diaminotriazine derivatives affect enantioselectively multiple herbicide target sites. Z. Naturforsch. C 2001, 56, 559–569. [Google Scholar]
- Parrish, M.D.; Unland, R.D.; Bertges, W.J. Introduction of Indaziflam for Weed Control in Fruit, Nut, and Grape Crops. North Central Weed Science Society Proceedings, Kansas City, MO, USA, 7–10 December 2009; [CD-ROM]. North Central Weed Sci. Soc.: Champaign, IL, USA, 2009. [Google Scholar]
- Kojima, H.; Hitomi, Y.; Numata, T.; Tanaka, C.; Imai, K.; Omokawa, H. Analysis of gene expression in rice root tips treated with R-1-α-methylbenzyl-3-p-tolylurea using PCR-based suppression subtractive hybridization. Pestic. Biochem. Physiol 2009, 93, 58–64. [Google Scholar]
- Kojima, H.; Numata, T.; Tadaki, R.; Omokawa, H. PCR-based suppression subtractive hybridization analyses of enantioselective gene expression in root tips of wheat treated with optically active urea compounds. Pestic. Biochem. Physiol 2010, 98, 359–369. [Google Scholar]
- Díaz-Cacho, P.; Moral, R.; Encina, A.; Acebes, J.L.; Álvarez, J. Cell wall modifications in bean (Phaseolus vulgaris) callus cultures tolerant to isoxaben. Physiol. Plant 1999, 107, 54–59. [Google Scholar]
- Encina, A.; Moral, R.M.; Acebes, J.L.; Álvarez, J.M. Characterization of cell walls in bean (Phaseolus vulgaris L.) callus cultures tolerant to dichlobenil. Plant Sci 2001, 160, 331–339. [Google Scholar]
- Schabenberger, O.; Tharp, B.E.; Kells, J.J.; Penner, D. Statistical tests for hormesis and effective dosages in herbicide dose response. Agron. J 1999, 91, 713–721. [Google Scholar]
- Calabrese, E.D.; Blain, R.B. Hormesis and plant biology. Environ. Pollut 2009, 157, 42–48. [Google Scholar]
- Belz, R.G.; Cedergreen, N.; Duke, S.O. Herbicide hormesis—Can it be useful in crop production? Weed Res 2011, 51, 321–332. [Google Scholar]
- Grossmann, K. Quinclorac belongs to a new class of highly selective auxin herbicides. Weed Sci 1998, 46, 707–716. [Google Scholar]
- Grossmann, K. Mode of action of auxin herbicides: A new ending to a long, drawn out story. Trends Plant Sci 2000, 5, 506–508. [Google Scholar]
- Sunohara, Y.; Matsumoto, H. Quinclorac-induced cell death is accompanied by generation of reactive oxygen species in maize root tissue. Phytochemistry 2008, 69, 2312–2319. [Google Scholar]
- Seifert, G.J.; Blaukopf, C. Irritable walls: The plant extracellular matrix and signaling. Plant Physiol 2010, 153, 467–478. [Google Scholar]
- Encina, A.; Sevillano, J.M.; Acebes, J.L.; Álvarez, J. Cell wall modifications of bean (Phaseolus vulgaris) cell suspensions during habituation and dehabituation to dichlobenil. Physiol. Plant 2002, 114, 182–191. [Google Scholar]
- Abdallah, I.; Fischer, A.J.; Elmore, C.L.; Saltveit, M.E.; Mohammed, Z. Mechanism of resistance to quinclorac in smooth crabgrass (Digitaria ischaemum). Pestic. Biochem. Physiol 2006, 84, 38–48. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar]
- Updegraff, D.M. Semi-micro determination of cellulose in biological materials. Anal. Biochem 1969, 32, 420–424. [Google Scholar]
- Saeman, J.F.; Moore, W.E.; Millet, M.A. Sugar Units Present. In Methods in Carbohydrate Chemistry; Whistler, R.L., Ed.; Academic Press: New York NY, USA, 1963; pp. 54–69. [Google Scholar]
- Dische, Z. Color Reactions of Carbohydrates. In Methods in Carbohydrate Chemistry; Whistler, R.L., Wolfrom, M.L., Eds.; Academic Press: New York NY, USA, 1962; pp. 475–514. [Google Scholar]
- Dugger, W.M.; Palmer, R.L. Incorporation of UDP-Glucose into cell wall glucans and lipids by intact cotton fibers. Plant Physiol 1986, 81, 464–470. [Google Scholar]
- Coimbra, M.A.; Delgadillo, I.; Waldron, K.W. Isolation and Analysis of Cell Wall Polymers from Olive Pulp. In Modern Methods of Plant Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin, Germany, 1996; pp. 19–44. [Google Scholar]
- Statistica Software, version 6.0; Statsoft Inc.: Tulsa, OK, USA, 2001.

 ), KOH (
), KOH (
 ), Sn-CR (
), Sn-CR (
 ), ANW (
), ANW (
 ) and α-Cellulose (■) from cell suspensions untreated (Control) or treated for 20 h with different CBIs at their I50 concentration (see Table 2). Data are expressed as total cpm incorporated into each fraction (A) and as percentages of incorporation in each fraction regarding to total incorporation into the cell wall (B). Values are means ± SD of three technical replicates.
) and α-Cellulose (■) from cell suspensions untreated (Control) or treated for 20 h with different CBIs at their I50 concentration (see Table 2). Data are expressed as total cpm incorporated into each fraction (A) and as percentages of incorporation in each fraction regarding to total incorporation into the cell wall (B). Values are means ± SD of three technical replicates.
 ), KOH (
), KOH (
 ), Sn-CR (
), Sn-CR (
 ), ANW (
), ANW (
 ) and α-Cellulose (■) from cell suspensions untreated (Control) or treated for 20 h with different CBIs at their I50 concentration (see Table 2). Data are expressed as total cpm incorporated into each fraction (A) and as percentages of incorporation in each fraction regarding to total incorporation into the cell wall (B). Values are means ± SD of three technical replicates.
) and α-Cellulose (■) from cell suspensions untreated (Control) or treated for 20 h with different CBIs at their I50 concentration (see Table 2). Data are expressed as total cpm incorporated into each fraction (A) and as percentages of incorporation in each fraction regarding to total incorporation into the cell wall (B). Values are means ± SD of three technical replicates.



| CBI | Chemical Name | References |
|---|---|---|
| Dichlobenil | 2,6-dichlorobenzonitrile | [5] |
| AE F150944 | N2-(1-ethyl-3-phenylpropyl)-6-(1-fluoro-1-methylethyl)-1,3,5-triazine-2,4-diamine | [6] |
| Flupoxam | 1-[4-chloro-3-[(2,2,3,3,3-pentafluoropropoxymethyl) phenyl]-5-phenyl-1H-1,2,4-triazole-3-carboximide | [7] |
| Triazofenamide | 1-(3-methyl phenyl)-5-phenyl-1H-1,2,4-3 triazole-3-carboximide | [8] |
| Compound 1 | 5-tert-butyl-carbamoyloxyl-3-(3-trifluoromethyl) phenyl-4-thiazolidinone | [9] |
| CGA 325′615 | 1-cyclohexyl-5-(2,3,4,5,6-pentafluorophenoxy)-1 λ4,2,4,6-thiatriazin-3-amine | [10] |
| Oxaziclomefone | 3-(1-(3,5-dichlorophenyl)-1-methylethyl)-3,4-dihydro-6-methyl-5-phenyl-2H-1,3-oxazin-4-one | [11,12] |
| Quinclorac | 3,7-dichloro-8-quinoline carboxylic acid | [13,14] |
| Inhibition Parameters | DWt/FWt | ||||||
|---|---|---|---|---|---|---|---|
| CBI | I10 | I50 | I90 | Active Concentration Range (I90/I10) | DWt/FWt (I50) | DWt/FWt (I90) | Cellulose (μg mg−1 CW) |
| CGA 325′615 | <0.1 nM | 0.5 nM | 10 nM | 100 | 0.048 | 0.052 | 241.7 ± 33.3 |
| AE F150944 | 0.8 nM | 1 nM | >20 mM | ~25000 | 0.053 | ~0.064 | 277.2 ± 18.7 * |
| Flupoxam | 0.2 nM | 2 nM | 400 nM | 2000 | 0.048 | 0.058 | 216.3 ± 5.5 |
| Triazofenamide | 4 nM | 15 nM | 100 nM | 25 | 0.047 | 0.046 | 258.5 ± 29.3 * |
| Oxaziclomefone | 0.6 nM | 30 nM | >1 μM | ~1667 | 0.048 | ~0.052 | 229 ± 22.7 |
| Dichlobenil | 0.2 μM | 0.5 μM | 1 μM | 5 | 0.032 | 0.048 | 220.4 ± 18.4 |
| Quinclorac | 4 μM | 10 μM | 20 μM | 5 | 0.050 | 0.053 | 247.9 ± 4.1 |
| Compound 1 | 20 nM | 20 μM | 200 μM | 10000 | 0.055 | 0.060 | 328.5 ± 37.2 * |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
García-Angulo, P.; Alonso-Simón, A.; Encina, A.; Álvarez, J.M.; Acebes, J.L. Cellulose Biosynthesis Inhibitors: Comparative Effect on Bean Cell Cultures. Int. J. Mol. Sci. 2012, 13, 3685-3702. https://doi.org/10.3390/ijms13033685
García-Angulo P, Alonso-Simón A, Encina A, Álvarez JM, Acebes JL. Cellulose Biosynthesis Inhibitors: Comparative Effect on Bean Cell Cultures. International Journal of Molecular Sciences. 2012; 13(3):3685-3702. https://doi.org/10.3390/ijms13033685
Chicago/Turabian StyleGarcía-Angulo, Penélope, Ana Alonso-Simón, Antonio Encina, Jesús M. Álvarez, and José L. Acebes. 2012. "Cellulose Biosynthesis Inhibitors: Comparative Effect on Bean Cell Cultures" International Journal of Molecular Sciences 13, no. 3: 3685-3702. https://doi.org/10.3390/ijms13033685