Fly Ash Porous Material using Geopolymerization Process for High Temperature Exposure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Behavior of Porous Geopolymer after Sintering
2.2. Compressive Strength
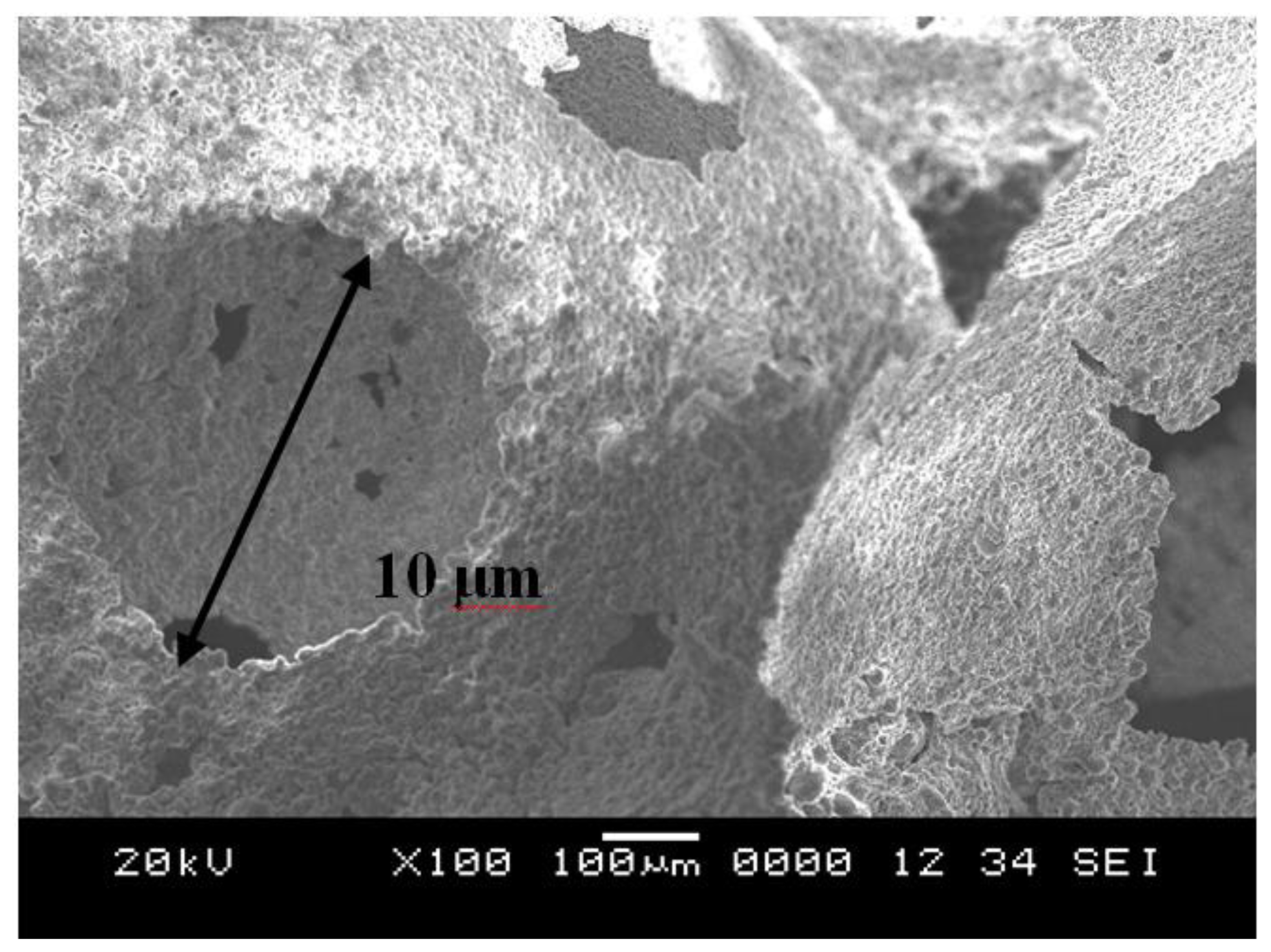
2.3. Scanning Electron Microscopy Analysis
2.4. Water Absorption and Porosity
3. Experimental Setup
3.1. Materials
3.2. Mixture Compositions
3.3. Samples Preparation
3.4. Curing Regime and High Temperature Exposure
3.5. Compressive Test
3.6. Microstructure Analysis Test
4. Conclusions
Acknowledgments
References
- Davidovits, J. Geopolymer cement to minimize carbon-dioxide greenhouse-warming. Ceram. Trans 1993, 37, 165–182. [Google Scholar]
- Davidovits, J. Global warming impact on the cement and aggregates industries. World Resour. Rev 1993, 6, 263–278. [Google Scholar]
- Mustafa Albakri, A.M.; Kamarudin, H.; Bnhussain, M.; Khairul Nizar, I.; Rafiza, A.R.; Zarina, Y. Microstructure of different NaOH molarity of fly ash-based green polymeric cement. J. Eng. Technol. Res 2011, 3, 44–49. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal 1991, 37, 1633–1656. [Google Scholar]
- Thomas Paul, K.; Satpthy, S.K.; Manna, I.; Chakraborty, K.K.; Nando, G.B. Preparation and characterization of nano structured materials from fly ash: A waste from thermal power stations, by high energy ball milling. Nanoscale Res. Lett 2007, 2, 397–404. [Google Scholar]
- Davidoits, J. High-alkali Cements for 21st Century Concretes. In Concrete Technology Past, Present and Future; Mehta, P.K., Ed.; American Concrete Institute: Detroit, MI, USA, 1994; Volume SP-144, pp. 383–397. [Google Scholar]
- Mustafa, Al; Bakri, A.M.; Kamarudin, H.; Karem, O.A.K.A.; Ruzaidi, C.M.; Rafiza, A.R.; Norazian, M.N. Optimization of /fly ash ratio on the compressive strength of manufacturing fly ash based geopolymer. J. Appl. Mechan. Mat 2011, 110–116, 734–739. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar]
- Davidoits, J. Geopolymer: Man-Made Rock Geosynthesis and the resulting development of very early high strength cement. J. Mat. Educ 1994, 16, 91–139. [Google Scholar]
- Davidovits, J. Chemistry of geopolymeric systems terminology, geopolymer 99. Geopolymer Int. Conf. Proc 1999, 11, 9–39. [Google Scholar]
- Allahverdi, A.; Skvara, F. Nitric acid attack on hardened paste of geopolymeric cement. Ceramic-Silikatay 2001, 45, 81–88. [Google Scholar]
- Perera, D.S.; Vance, E.R.; Aly, Z.; Finnie, K.S.; Hanna, J.V.; Nicholson, C.L.; Trautman, R.L.; Stewart, M.W.A. Characterisation of Geopolymer for the Immobilisation of Intermediate Level Waste. Proceedings of ASME 2003 9th International Conference on Radioactive Waste Management and Environmental Remediation, Oxford, England, 21–25 Sepember 2003.
- Daniel, L.Y.K.; Sanjayan, J.G. Damage behavior of geopolymer composites exposed to elevated temperatures. Cement Concr. Compos 2008, 30, 986–991. [Google Scholar]
- Chew, M.Y.L. Assessment of fire damaged concrete. Build Environ 1993, 28, 97–102. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry & Application, 3rd ed; Institut Geopolymere: Paris, France, 2008. [Google Scholar]
- Sathia, R.; Babu, K.G.; Santhanam, M. Durability study of low calcium fly ash geopolymer concrete. Proceedings of the 3rd ACF International Conference- ACF/VCA, Ho Chi Minh City, Vietnam, 11–13 November 2008.
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly ash and kaolinite based geopolymers. Chem. Eng. J 2002, 89, 63–73. [Google Scholar]
- Xu, H.; Deventer, J. The geopolymerisation of alumino-silicate minerals. Int. J. Min. Proc 2000, 59, 247–266. [Google Scholar]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly-Ash-Based Geopolymer Concrete; Research Report GC 1; Faculty of Engineering, Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- Perera, D.S.; Trautman, R.L. Geopolymers with the potential for use as refractory castables. Adv. Technol. Mat. Mat. Proc. J 2006, 2, 187–189. [Google Scholar]
- Hardjito, D.; Cheak, C.C.; Lee, C.H. Strength and setting times of low calcium fly ash- based geopolymer mortar. Mod. Appl. Sci 2008, 2, 3–11. [Google Scholar]
- Rangan, B.V. Low-Calcium, Fly-Ash-Based Geopolymer Concrete. In Concrete Construction Engineering Handbook; Taylor and Francis Group: Boca Raton, FL, USA, 2008; pp. 1–19. [Google Scholar]



| Ratio Na2SiO3/NaOH | Mass reduction (%) | ||
|---|---|---|---|
| 600 °C | 800 °C | 1000 °C | |
| 2.5 | 17.32 | 19.45 | 22.36 |
| 3.0 | 17.80 | 19.50 | 22.65 |
| 3.5 | 17.95 | 19.70 | 27.35 |
| Ratio Na2SiO3/NaOH | Compressive strength after 3 days (Mpa) | |||
|---|---|---|---|---|
| unsintered | 600 °C | 800 °C | 1000 °C | |
| 2.5 | 12.12 | 10.41 | 8.90 | 8.63 |
| 3.0 | 15.56 | 10.04 | 24.00 | 22.83 |
| 3.5 | 21.99 | 14.63 | 24.33 | 42.40 |
| Mixture of geopolymer | Mass ratios (2.5) | Mass ratios (3.0) | Mass ratios (3.5) |
|---|---|---|---|
| Ratio of fly ash/alkaline activator | 2.5 | 3.0 | 3.5 |
| Ratio Na2SiO3/NaOH | 2.5 | 3.0 | 3.5 |
| Mass of fly ash (g) | 1210 | 1270 | 1310 |
| Mass of NaOH (g) | 137.8 | 105.5 | 83.3 |
| Mass of Na2SiO3 (g) | 344.4 | 316.4 | 291.7 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdullah, M.M.A.B.; Jamaludin, L.; Hussin, K.; Bnhussain, M.; Ghazali, C.M.R.; Ahmad, M.I. Fly Ash Porous Material using Geopolymerization Process for High Temperature Exposure. Int. J. Mol. Sci. 2012, 13, 4388-4395. https://doi.org/10.3390/ijms13044388
Abdullah MMAB, Jamaludin L, Hussin K, Bnhussain M, Ghazali CMR, Ahmad MI. Fly Ash Porous Material using Geopolymerization Process for High Temperature Exposure. International Journal of Molecular Sciences. 2012; 13(4):4388-4395. https://doi.org/10.3390/ijms13044388
Chicago/Turabian StyleAbdullah, Mohd Mustafa Al Bakri, Liyana Jamaludin, Kamarudin Hussin, Mohamed Bnhussain, Che Mohd Ruzaidi Ghazali, and Mohd Izzat Ahmad. 2012. "Fly Ash Porous Material using Geopolymerization Process for High Temperature Exposure" International Journal of Molecular Sciences 13, no. 4: 4388-4395. https://doi.org/10.3390/ijms13044388





