Quercetin and Epigallocatechin Gallate Induce in Vitro a Dose-Dependent Stiffening and Hyperpolarizing Effect on the Cell Membrane of Human Mononuclear Blood Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biochemical and Inflammatory Profile of the Selected Patients
2.2. Evaluation of Q and EGCG Effect on PBMCs Membrane Parameters
2.2.1. Preliminary Experiments
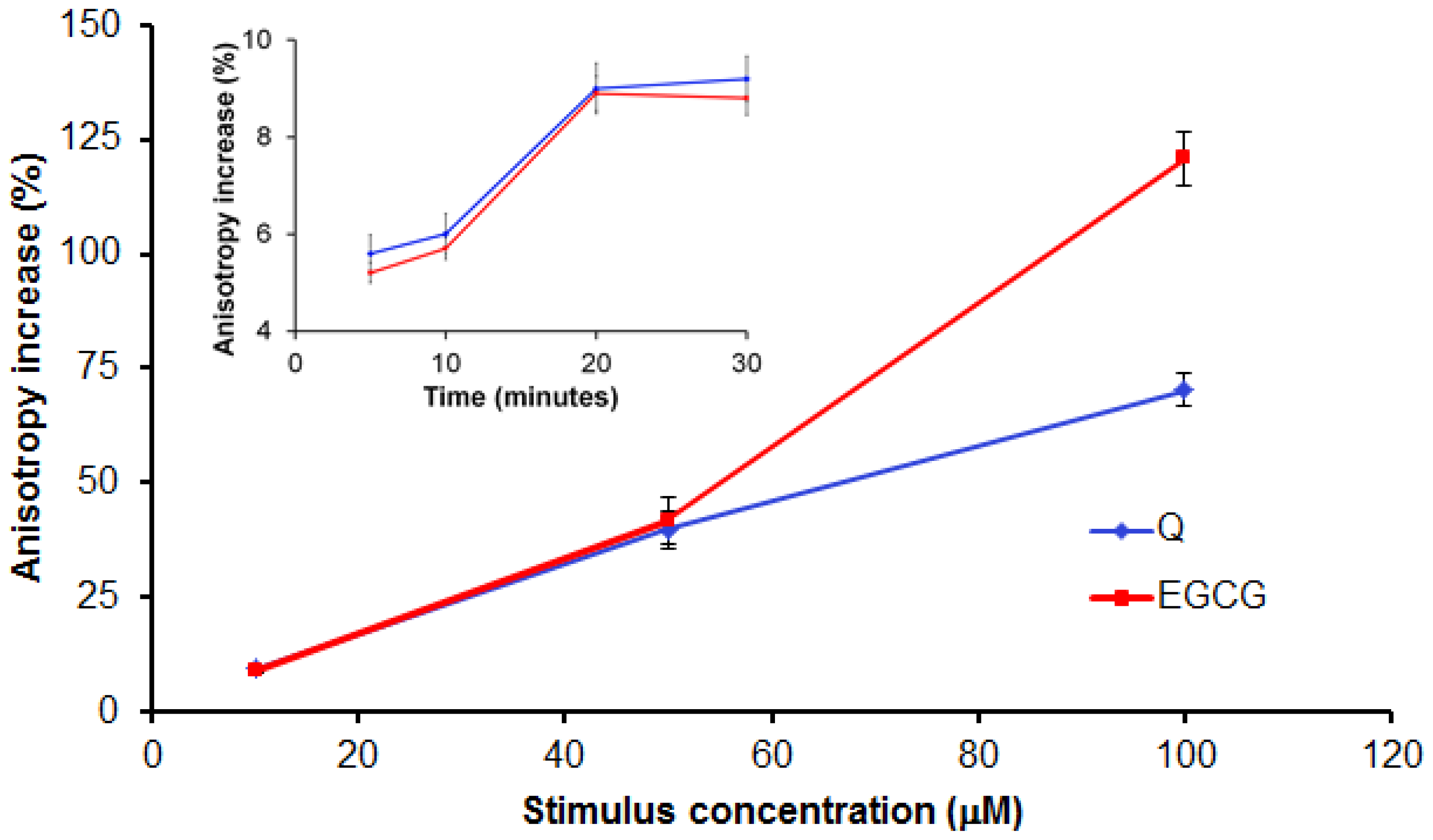
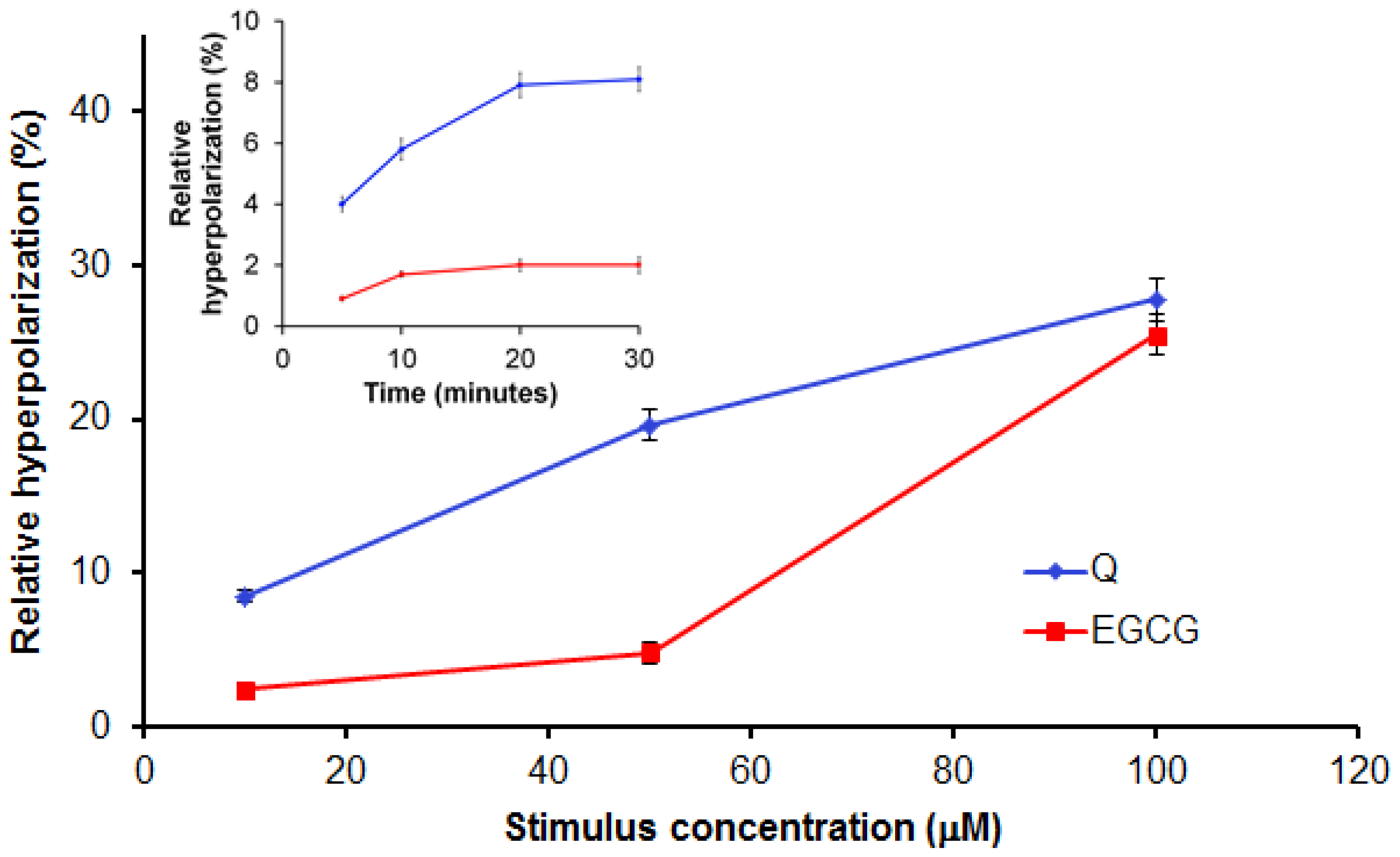
2.2.2. Evaluation of Q and EGCG Concentration Effect on PBMC Membrane Parameters
2.2.3. Effect of 10 μM of Q and EGCG on PBMCs Membrane Properties
2.2.4. Discussion
3. Experimental Section
3.1. Materials and Devices
3.2. Study Design
- NGNC subgroup, n = 15, included normoglycemia (fasting plasma glucose, Gli < 110 mg/dL) normocholesterolemia (total cholesterol, TC < 200 mg/dL) subjects.
- NGHC subgroup, n = 10, included normoglycemia-hypecholesterolemia (TC > 200 mg/dL) patients.
- HGNC subgroup, n = 12, included hyperglycemia (Gli > 110 mg/dL) normocholesterolemia patients.
- HGHC subgroup, n = 14, included hyperglycemia-hypercholesterolemia patients.
3.3. Biological Samples
3.4. Methods
3.4.1. Fluorescence Anisotropy Measurements
3.4.2. Transmembrane Potential Evaluation
3.4.3. Statistical Analysis
4. Conclusions
Acknowledgments
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate transaminase |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DMSO | dimethylsulfoxide |
| EGCG | epigallocatechin gallate |
| Gli | fasting plasma glucose |
| HbA1c | glycated haemoglobin |
| HOMA-IR | homeostatic model analysis for insulin resistance |
| ICAM-1 | intercellular adhesion molecule 1 |
| MCP-1 | monocytechemoatractant protein-1 |
| PBMCs | peripheral blood mononuclear cells |
| Q | quercetin |
| T2D | type 2 diabetes mellitus |
| TC | total cholesterol |
| TG | triglycerides |
| TMA-DPH | 1-(4-trimethylammonium-phenyl)-6-phenyl-1,3,5-hexatriene p-toluensulfonate |
| DiBAC4(3) | bis-(1,3-dibutylbarbituric acid) trimethineoxonol. |
References
- Boots, A.W.; Haenen, G.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol 2008, 585, 325–337. [Google Scholar]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med 2010, 31, 435–445. [Google Scholar]
- Cheplick, S.; Kwon, Y.I.; Bhowmik, P.; Shetty, K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresour. Technol 2010, 101, 404–413. [Google Scholar]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Tuomainen, T.P.; Kurl, S.; Salonen, J.T. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: The kuopio ischaemic heart disease risk factor study. Br. J. Nutr 2008, 100, 890–895. [Google Scholar]
- Erlejman, A.G.; Jaggers, G.; Fraga, C.G.; Oteiza, P.I. TNFα-induced NF-κB activation and cell oxidant production are modulated by hexamericprocyanidins in Caco-2 cells. Arch. Biochem. Biophys 2008, 476, 186–195. [Google Scholar]
- Verstraeten, S.V.; Mackenzie, G.G.; Oteiza, P.I.; Fraga, C.G. (−)-Epicatechin and related procyanidins modulate intracellular calcium and prevent oxidation in Jurkat T cells. Free Radic. Res 2008, 42, 864–872. [Google Scholar]
- Fujimura, Y.; Tachibana, H.; Kumai, R.; Yamada, K. A difference between epigallocatechin-3-gallate and epicatechin-3-gallate on anti-allergic effect is dependent on their distinct distribution to lipid rafts. Biofactors 2004, 21, 133–135. [Google Scholar]
- Tachibana, H.; Fujimura, Y.; Yamada, K. Tea polyphenol epigallocatechin-3-gallate associates with plasma membrane lipid rafts: Lipid rafts mediate anti-allergic action of the catechin. Biofactors 2004, 21, 383–385. [Google Scholar]
- Maldonado-Celis, M.E.; Bousserouel, S.; Gosse, F.; Lobstein, A.; Raul, F. Apple procyanidins activate apoptotic signaling pathway in human colon adenocarcinoma cells by a lipid-raft independent mechanism. Biochem. Biophys. Res. Commun 2009, 388, 372–376. [Google Scholar]
- Annaba, F.; Kumar, P.; Dudeja, A.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. Am. J. Physiol. Gastrointest. Liver Physiol 2010, 298, G467–G473. [Google Scholar]
- Liang, X.J.; Yin, J.J.; Zhou, J.W.; Wang, P.C.; Taylor, B.; Cardarelli, C.; Kozar, M.; Forte, R.; Aszalos, A.; Gottesman, M.M. Changes in biophysical parameters of plasma membranes influence cisplatin resistance of sensitive and resistant epidermal carcinoma cells. Exp. Cell Res 2004, 293, 283–291. [Google Scholar]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Yasuda, Y.; Kubota, M.; Adachi, S.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechingallate inhibits growth and activation of the VEGF/VEGFR axis in human colorectal cancer cells. Chem. Biol. Interact 2010, 185, 247–252. [Google Scholar]
- Tribolo, S.; Lodi, F.; Connor, C.; Suri, S.; Wilson, V.G.; Taylor, M.A.; Needs, P.A.; Kroon, P.A.; Hughes, D.A. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis 2008, 197, 50–56. [Google Scholar]
- Winterbone, M.S.; Tribolo, S.; Needs, P.W.; Kroon, P.A.; Hughes, D.A. Physiologically relevant metabolites of quercetin have no effect on adhesion molecule or chemokine expression in human vascular smooth muscle cells. Atherosclerosis 2009, 202, 431–438. [Google Scholar]
- Van Blitterswijk, W.J.; van Hoeven, R.P.; van der Meer, B.W. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim. Biophys. Acta 1981, 644, 323–332. [Google Scholar]
- Caimi, G.; Canino, B.; Montana, M.; Ventimiglia, G.; Catania, A.; Lo Presti, R. Polymorphonuclear leukocyte membrane fluidity and cytosolic Ca2+ concentration in diabetes mellitus. Acta Diabetol 1998, 35, 158–160. [Google Scholar]
- Zimmermann, R.; Küttner, D.; Renner, L.D.; Kaufmann, M.; Werner, C. Fluidity modulation of phospholipid bilayers by electrolyte ions: Insights from fluorescence microscopy and microslit electrokinetic experiments. J. Phys. Chem. A 2012. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol 2005, 25, 29–38. [Google Scholar]
- Gutierrez, J.; Ballinger, S.W.; Darley-Usmar, V.M.; Landar, A. Free radicals, mitochondria, and oxidized lipids: The emerging role in signal transduction in vascular cells. Circ. Res 2006, 99, 924–932. [Google Scholar]
- Sánchez-Gallego, J.I.; López-Revuelta, A.; Sardina, J.L.; Hernández-Hernández, A.; Sánchez-Yagüe, J.; Llanillo, M. Membrane cholesterol contents modify the protective effects of quercetin and rutin on integrity and cellular viability in oxidized erythrocytes. Free Radic. Biol. Med 2010, 48, 1444–1454. [Google Scholar]
- Hon, G.M.; Hassan, M.S.; van Rensburg, S.J.; Abel, S.; Erasmus, R.T.; Matsha, T. Peripheral blood mononuclear cell membrane fluidity and disease outcome in patients with multiple sclerosis. Indian J. Hematol. Blood Transfus 2011, 28, 1–6. [Google Scholar]
- Breusing, N.; Grune, T.; Andrisic, L.; Atalay, M.; Bartosz, G.; Biasi, F.; Borovic, S.; Bravo, L.; Casals, I.; Casillas, R.; et al. An inter-laboratory validation of methods of lipid peroxidation measurement in UVA-treated human plasma samples. Free Radic. Res 2010, 44, 1203–1215. [Google Scholar]
- Kew, S.; Banerjee, T.; Minihane, A.M.; Finnegan, Y.E.; Williams, C.M.; Calder, P.C. Relation between the fatty acid composition of peripheral blood mononuclear cells and measures of immune cell function in healthy, free-living subjects aged 25–72 years. Am. J. Clin. Nutr 2003, 77, 1278–1286. [Google Scholar]
- Klapperstück, T.; Glanz, D.; Klapperstück, M.; Wohlrab, J. Methodological aspects of measuring absolute values of membrane potential in human cells by flow cytometry. Cytom. A 2009, 75, 593–608. [Google Scholar]
- Sergent, O.; Pereira, M.; Belhomme, C.; Chevanne, M.; Huc, L.; Lagadic-Gossmann, D. Role for membrane fluidity in ethanol-induced oxidative stress of primary rat hepatocytes. J. Pharmacol. Exp. Ther 2005, 313, 104–111. [Google Scholar]
- Sergent, O.; Ekroos, K.; Lefeuvre-Orfila, L.; Rissel, M.; Forsberg, G.B.; Oscarsson, J.; Andersson, T.B.; Lagadic-Gossmann, D. Ximelagatran increases membrane fluidity and changes membrane lipid composition in primary human hepatocytes. Toxicol. Vitr 2009, 23, 1305–1310. [Google Scholar]
- Van Aalst, J.A.; Burmeister, W.; Fox, P.L.; Graham, L.M. α-tocopherol preserves endothelial cell migration in the presence of cell-oxidized low-density lipoprotein by inhibiting changes in cell membrane fluidity. J. Vasc. Surg 2004, 39, 229–237. [Google Scholar]
- Ghosh, P.K.; Vasanji, A.; Murugesan, G.; Eppell, S.J.; Graham, L.M.; Fox, P.L. Membrane microviscosity regulates endothelial cell motility. Nat. Cell. Biol 2002, 4, 894–900. [Google Scholar]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr 2012, 52, 213–248. [Google Scholar]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromol. Med 2008, 10, 236–246. [Google Scholar]
- Vargas, A.J.; Burd, R. Hormesis and synergy: Pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev 2010, 68, 418–428. [Google Scholar]
- Galluzzo, P.; Martini, C.; Bulzomi, P.; Leone, S.; Bolli, A.; Pallottini, V.; Marino, M. Quercetin-induced apoptotic cascade in cancer cells: Antioxidant versus estrogen receptor α-dependent mechanisms. Mol. Nutr. Food Res 2009, 53, 699–708. [Google Scholar]
- Tang, X.; Zhu, X.; Liu, S.; Nicholson, R.C.; Ni, X. Phytoestrogens induce differential estrogen receptor β-mediated responses in transfected MG-63 cells. Endocrine 2008, 34, 29–35. [Google Scholar]
- Sotoca, A.M.; Ratman, D.; van der Saag, P.; Ström, A.; Gustafsson, J.A.; Vervoort, J.; Rietjens, I.M.C.M.; Murk, A.J. Phytoestrogen mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERα/ERβ ratio. J. Steroid Biochem. Mol. Biol 2008, 112, 171–178. [Google Scholar]
- Phiel, K.L.; Henderson, R.A.; Adelman, S.J.; Merle Elloso, M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett 2005, 97, 107–113. [Google Scholar]
- Pierdominici, M.; Maselli, A.; Colasanti, T.; Giammarioli, A.M.; Delunardo, F.; Vacirca, D.; Sanchez, M.; Giovannetti, A.; Malorni, W.; Ortona, E. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol. Lett 2010, 132, 79–85. [Google Scholar]
- Tong, P.; Thomas, T.; Berrish, T.; Humphriss, D.; Barriocanal, L.; Stewart, M.; Walker, M.; Wilkinson, R.; Alberti, K.G. Cell membrane dynamics and insulin resistance in non-insulin-dependent diabetes mellitus. Lancet 1995, 345, 357–358. [Google Scholar]
- Masuda, M.; Murakami, T.; Egawa, H.; Murata, K. Decreased fluidity of polymorphonuclear leukocyte membrane in streptozocin-induced diabetic rats. Diabetes 1990, 39, 466–470. [Google Scholar]
- Kantar, A.; Giorgi, P.L.; Curatola, G.; Fiorini, R. Alterations in membrane fluidity of diabetic polymorphonuclear leukocytes. Biochem. Med. Metab. Biol 1991, 46, 422–426. [Google Scholar]
- Ceriello, A.; Quagliaro, L.; Piconi, L.; Assaloni, R.; DaRos, R.; Maier, A.; Esposito, K.; Giugliano, D. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes 2004, 53, 701–710. [Google Scholar]
- Tesch, G.H. MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am. J. Physiol. Ren. Physiol 2008, 294, F697–F701. [Google Scholar]
- Ford, E.S. The metabolic syndrome and C-reactive protein, fibrinogen, and leukocyte count: Findings from the third national health and nutrition examination survey. Atherosclerosis 2003, 168, 351–358. [Google Scholar]
- Hoefle, G.; Saely, C.H.; Risch, L.; Koch, L.; Schmid, F.; Rein, P.; Aczél, S.; Berchtold, S.; Drexel, H. Relationship between the adipose-tissue hormone resistin and coronary artery disease. Clin. Chim. Acta 2007, 386, 1–6. [Google Scholar]
- Boström, E.A.; Tarkowski, A.; Bokarewa, M. Resistin is stored in neutrophil granules being released upon challenge with inflammatory stimuli. Biochim. Biophys. Acta 2009, 1793, 1894–1900. [Google Scholar]
- Veskoukis, A.S.; Tsatsakis, A.M.; Kouretas, D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones 2012, 17, 11–21. [Google Scholar]
- Matsuda, M. Measuring and estimating insulin resistance in clinical and research settings. Nutr. Metab. Cardiovasc. Dis 2010, 2, 79–86. [Google Scholar]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar]
- Nash, G.B.; Jones, J.G.; Mikita, J.; Dormandy, J.A. Methods and theory for analysis of flow of white cell subpopulations through micropore filters. Br. J. Haematol 1988, 70, 165–170. [Google Scholar]
- Margina, D.; Ilie, M.; Gradinaru, D.; Vladica, M.; Pencea, C.; Mitrea, N.; Katona, E. Redox status parameters and PBMC membrane fluidity in diabetes mellitus. Clin. Exp. Med. J 2009, 3, 279–291. [Google Scholar]
- Ilie, M.; Margina, D.; Nisiparu, L.; Ganea, C.; Katona, E. Flavonoids effect on the lipid order parameter of peripheral blood mononuclear cells. Rom. J. Biophys 2009, 19, 38–43. [Google Scholar]
- Pebay-Peyroula, E.; Dufourc, E.J.; Szabo, A.G. Location of diphenyl-hexatriene and trimethylammonium-diphenyl-hexatriene in dipalmitoylphosphatidylcholine bilayers by neutron diffraction. Biophys. Chem 1994, 53, 45–56. [Google Scholar]
- Martins, A.P.; Lopes, P.A.; Martins, S.V.; Madeira, A.; Santos, N.C.; Prates, J.A.M.; Moura, T.F.; Soveral, G. Conjugated linoleic acid reduces permeability and fluidity of adipose plasma membranes from obese Zucker rats. Biochem. Biophys. Res. Commun 2010, 398, 199–204. [Google Scholar] [Green Version]
- Huang, Z.J.; Haugland, R.P. Partition coefficients of fluorescent probes with phospholipid membranes. Biochem. Biophys. Res. Commun 1991, 181, 166–171. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Kluwer Academic/Plenum Press: New York, NY, USA, 1999; p. 299. [Google Scholar]






| Biochemical parameters | NGNC (n = 15) | NGHC (n = 10) | HGNC (n = 12) | HGHC (n = 14) |
|---|---|---|---|---|
| Gli (mg/dL) | 94.95 ± 8.25 | 90.74 ± 9.11 | 172.33 ± 82.07 *,a | 205.48 ± 115.21 *,a |
| HbA1c (%) | 5.26 ± 0.45 | 5.80 ± 0.54 | 7.80 ± 1.67 *,a | 9.55 ± 0.21 *,a |
| TC (mg/dL) | 167.29 ± 21.78 | 226.67 ± 16.72 *,b | 164.25 ± 22.92 | 232.91 ± 39.92 *,b |
| TG (mg/dL) | 92.50 ± 39.92 | 123.11 ± 74.39 | 134.17 ± 38.32 | 146.10 ± 45.56 |
| HDL (mg/dL) | 43.86 ± 9.69 | 49.83 ± 6.68 | 35.63 ± 8.21 | 52.39 ± 13.51 |
| LDL (mg/dL) | 104.14 ± 17.73 | 148.67 ± 11.68 *,b | 91.60 ± 34.51 | 142.70 ± 24.21 *,b |
| AST (U/L) | 24.43 ± 12.15 | 21.50 ± 4.11 | 20.90 ± 9.49 | 23.57 ± 8.67 |
| ALT (U/L) | 26.71 ± 23.24 | 20.00 ± 10.72 | 28.90 ± 14.55 | 34.71 ± 27.56 |
| Urea (mg/dL) | 29.62 ± 7.52 | 31.46 ± 12.82 | 51.20 ± 29.67 | 32.80 ± 19.31 |
| Creatinine (mg/dL) | 0.97 ± 0.21 | 0.80 ± 0.21 | 1.33 ± 0.94 | 0.93 ± 0.24 |
| Uric acid (mg/dL) | 5.57 ± 1.51 | 4.06 ± 1.01 | 5.44 ± 1.59 | 4.97 ± 1.25 |
| Parameter | NG (n = 25) | HG (n = 26) |
|---|---|---|
| CRP (mg/L) | 1.64 ± 1.47 | 2.19 ± 2.84 |
| ICAM-1 (ng/mL) | 3.53 ± 1.14 | 3.77 ± 2.07 |
| MCP-1 (pg/mL) | 156.63 ± 73.66 | 218.83 ± 107.58 * |
| Insulin (μIU/mL) | 11.99 ± 1.87 | 15.12 ± 10.82 |
| Resistin (ng/mL) | 103.39 ± 45.15 | 132.80 ± 29.42 * |
| HOMA-IR | 2.80 ± 0.64 | 6.90 ± 4.71 * |
| Inflammatory markers | NGNC (n = 15) | NGHC (n = 10) | HGNC (n = 12) | HGHC (n = 14) |
|---|---|---|---|---|
| CRP (mg/L) | 1.55 ± 1.36 | 1.84 ± 1.78 | 1.42 ± 1.38 | 2.86 ± 3.59 |
| ICAM-1 (ng/mL) | 3.44 ± 1.08 | 3.66 ± 1.29 | 3.91 ± 1.84 | 3.66 ± 2.31 |
| MCP-1 (pg/mL) | 121.11 ± 47.78 | 206.36 ± 76.54 * | 177.27 ± 85.75 | 254.45 ± 114.35 * |
| Parameter | NGNC (n = 15) | NGHC (n = 10) | HGNC (n = 12) | HGHC (n = 14) |
|---|---|---|---|---|
| Resistin (ng/mL) | 95.29 ± 47.46 | 111.50 ± 43.96 | 128.86 ± 28.49 * | 136.03 ± 31.15 * |
| HOMA-IR | 2.96 ± 0.73 | 2.54 ± 0.38 | 6.53 ± 4.81 * | 7.27 ± 4.81 * |
| Insulin (μIU/mL) | 12.50 ± 2.20 | 11.24 ± 0.88 | 14.38 ± 3.79 | 15.73 ± 14.54 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Margina, D.; Ilie, M.; Gradinaru, D. Quercetin and Epigallocatechin Gallate Induce in Vitro a Dose-Dependent Stiffening and Hyperpolarizing Effect on the Cell Membrane of Human Mononuclear Blood Cells. Int. J. Mol. Sci. 2012, 13, 4839-4859. https://doi.org/10.3390/ijms13044839
Margina D, Ilie M, Gradinaru D. Quercetin and Epigallocatechin Gallate Induce in Vitro a Dose-Dependent Stiffening and Hyperpolarizing Effect on the Cell Membrane of Human Mononuclear Blood Cells. International Journal of Molecular Sciences. 2012; 13(4):4839-4859. https://doi.org/10.3390/ijms13044839
Chicago/Turabian StyleMargina, Denisa, Mihaela Ilie, and Daniela Gradinaru. 2012. "Quercetin and Epigallocatechin Gallate Induce in Vitro a Dose-Dependent Stiffening and Hyperpolarizing Effect on the Cell Membrane of Human Mononuclear Blood Cells" International Journal of Molecular Sciences 13, no. 4: 4839-4859. https://doi.org/10.3390/ijms13044839





