A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of EPO Loading on the Tensile Properties of Blends
2.2. Effect of EPO Loading on the Flexural Properties of Blends
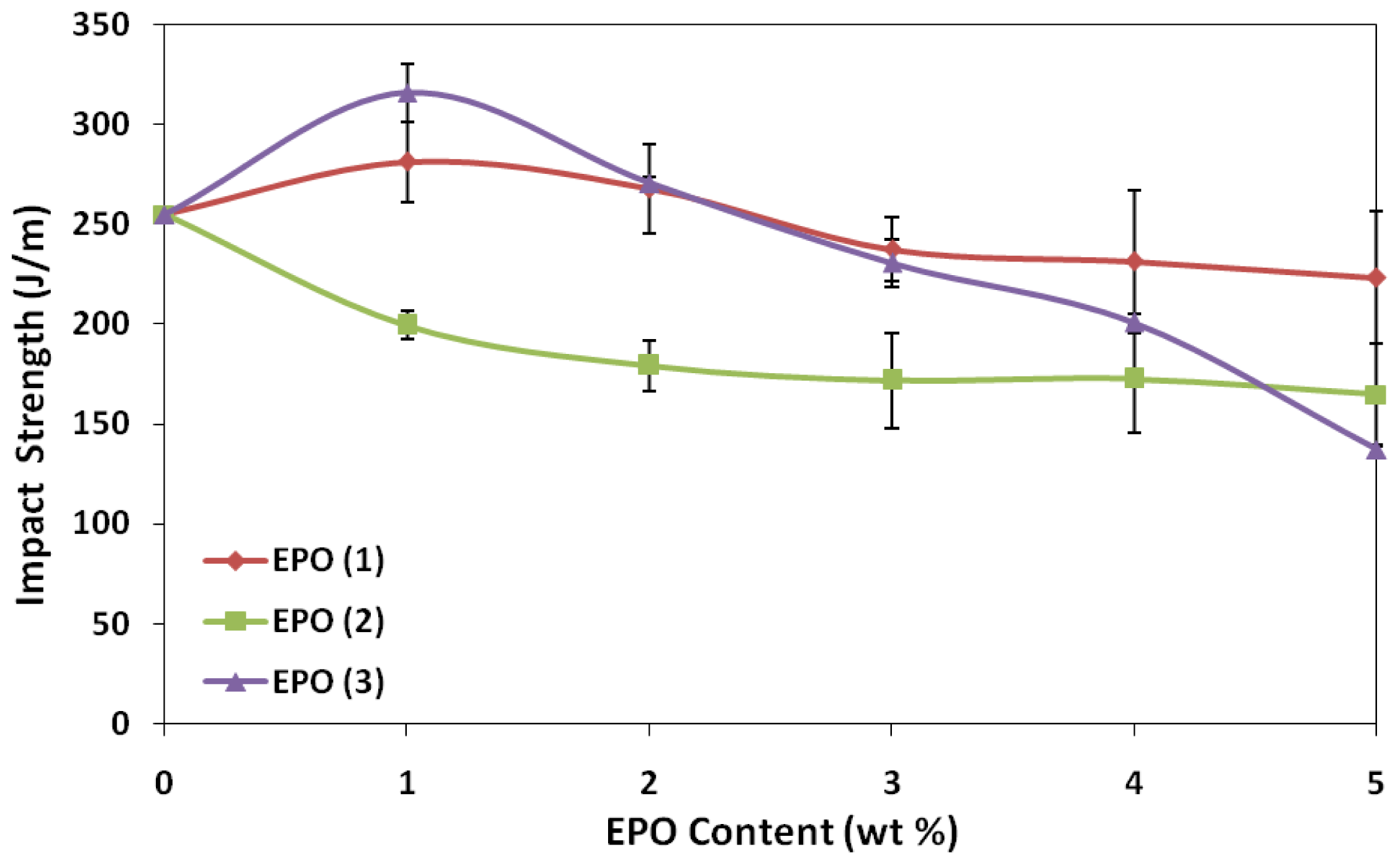
2.3. Effect of EPO Loading on the Impact Properties of Blends
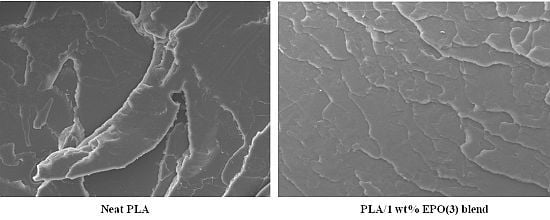
2.4. Morphological Study of PLA/EPO Blends
2.5. Dynamic Mechanical Analysis of PLA/EPO Blends
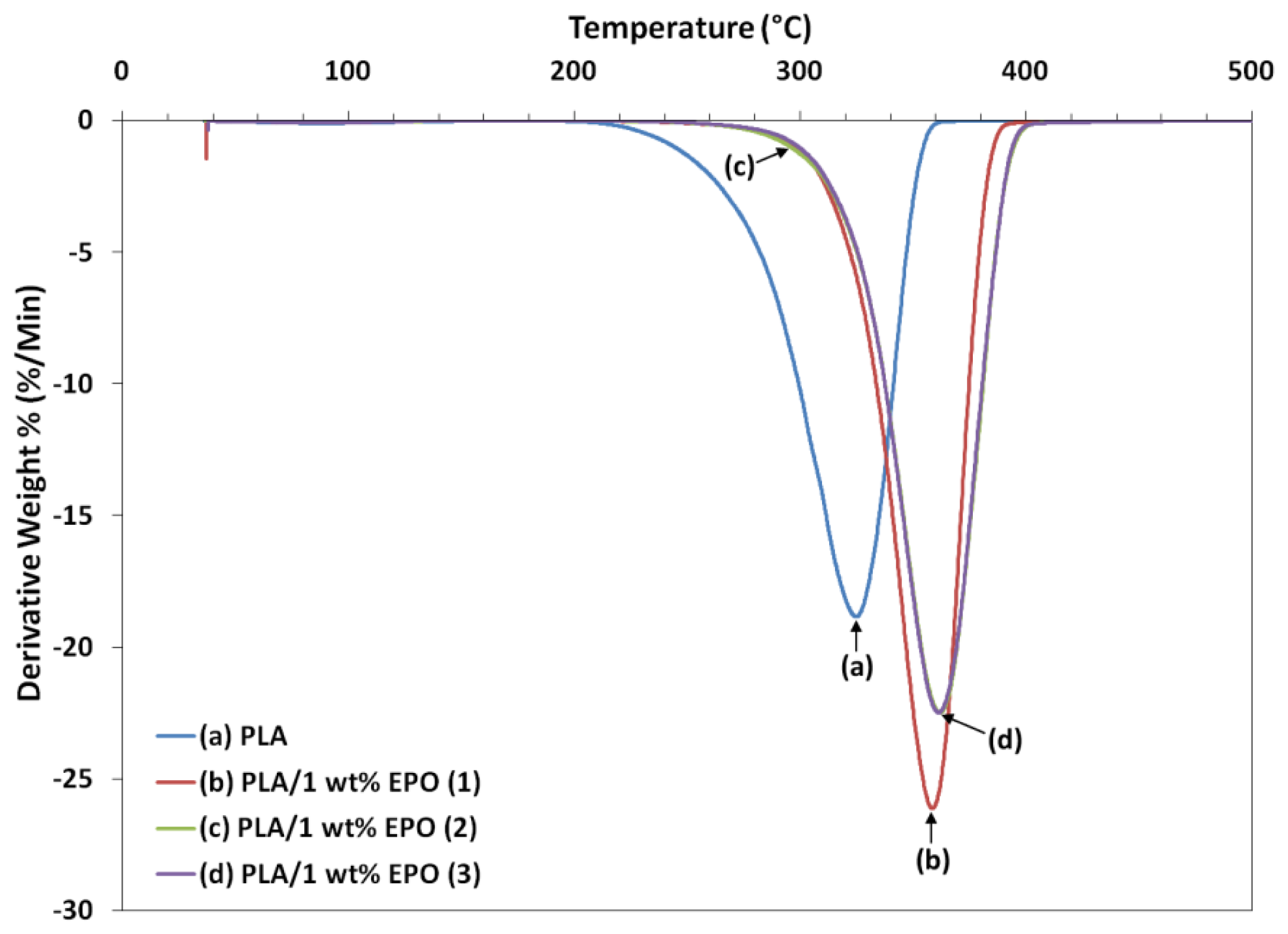
2.6. Thermogravimetric Study of PLA/EPO Blends
2.7. Differential Scanning Calorimetry Study of PLA/EPO Blends
2.8. X-Ray Diffraction (XRD) Study of PLA/EPO Blends
3. Experimental
3.1. Materials
3.2. Preparation of PLA/EPO Blends
3.3. Tensile Test
3.4. Flexural Test
3.5. Izod Impact Test
3.6. Scanning Electron Microscopy (SEM)
3.7. Dynamic Mechanical Analysis (DMA)
3.8. Thermogravimetric Analysis (TGA)
3.9. Differential Scanning Calorimetry (DSC)
3.10. X-Ray Diffraction (XRD) Analysis
4. Conclusions
Acknowledgments
References
- Kim, I.H.; Lee, S.C.; Jeong, Y.G. Tensile behavior and structural evolution of poly(lactic acid) monofilaments in glass transition region. Fibers Polym 2009, 10, 687–693. [Google Scholar]
- Kim, S.Y.; Shin, K.S.; Lee, S.H.; Kim, K.W.; Youn, J.R. Unique crystallization behavior of multi-walled carbon nanotube filled poly(lactic acid). Fibers Polym 2010, 11, 1018–1023. [Google Scholar]
- Shi, Q.; Chen, C.; Gao, L.; Jiao, L.; Xu, H.; Guo, W. Physical and degradation properties of binary or ternary blends composed of poly(lactic acid), thermoplastic starch and GMA grafted POE. Polym. Degrad. Stab 2011, 96, 175–182. [Google Scholar]
- Koo, G.H.; Jang, J. Surface modification of poly(lactic acid) by UV/ozone irradiation. Fibers Polym 2008, 9, 674–678. [Google Scholar]
- Groot, W.J.; Borén, T. Life cycle assessment of the manufacture of lactide and PLA biopolymers from sugarcane in Thailand. Int. J. Life Cycle Assess 2010, 15, 970–984. [Google Scholar]
- Matsumoto, K.; Taguchi, S. Enzymatic and whole-cell synthesis of lactate-containing polyesters: Toward the complete biological production of polylactate. Appl. Microbiol. Biotechnol 2010, 85, 921–932. [Google Scholar]
- Lee, S.H.; Song, W.S. Enzymatic hydrolysis of polylactic acid fiber. Appl. Biochem. Biotechnol 2010, 164, 89–102. [Google Scholar]
- Ljungberg, N.; Wesslén, B. Preparation and properties of plasticized poly(lactic acid) films. Biomacromolecules 2005, 6, 1789–1796. [Google Scholar]
- Ali, F.; Chang, Y.W.; Kang, S.C.; Yoon, J.Y. Thermal, mechanical and rheological properties of poly(lactic acid)/epoxidized soybean oil blends. Polym. Bull 2009, 62, 91–98. [Google Scholar]
- Emad, A.J.A.; Wan Md. Zin, W.Y.; Nor Azowa, I.; Mohd Zaki, A.R. Properties of epoxidized palm oil plasticized polytlactic acid. J. Mater. Sci 2010, 45, 1942–1946. [Google Scholar]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar]
- Martino, V.P.; Ruseckaite, R.A.; Jiméne, A. Thermal and mechanical characterization of plasticized poly(L-Lactide-co-L,D-Lactide) films for food packaging. J. Therm. Anal. Calorim 2006, 86, 707–712. [Google Scholar]
- Laurent, M.M. Rigid PVC/(layered silicate) nanocomposites produced through a novel melt-blending approach. J. Vinyl Addit. Technol 2009, 15, 77–86. [Google Scholar]
- Siemann, U. Solvent cast technology—A versatile tool for thin film production. Prog. Colloid Polym. Sci 2005, 130, 1–14. [Google Scholar]
- Lee, J.T.; Kim, M.W.; Song, Y.S.; Kang, T.J.; Youn, J.R. Mechanical properties of denim fabric reinforced poly(lactic acid). Fibers Polym 2010, 11, 60–66. [Google Scholar]
- Charles, E.W.; Charles, A.D.; James, W.S.; Mark, T.B. PVC Handbook; Hanser Publications: Cincinnati, OH, USA, 2005; pp. 174–175. [Google Scholar]
- Wypych, G. Handbook of Plasticizers; ChemTec Publishing: Toronto, Canada, 2004; pp. 112–117. [Google Scholar]
- Díez-Pascual, A.M.; Naffakh, M.; Gómez, M.A.; Marco, C.; Ellis, G.; González-Domínguez, J.M.; Ansón, A.; Martínez, M.T.; Martínez-Rubi, Y.; Simard, B.; et al. The influence of a compatibilizer on the thermal and dynamic mechanical properties of PEEK/carbon nanotube composites. Nanotechnology 2009, 20, 1–13. [Google Scholar]
- Seung-Hwan, L.; Siqun, W. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part A 2006, 37, 80–91. [Google Scholar]
- Adebiyi, A.P.; Adebiyi, A.O.; Jin, D.H.; Ogawa, T.; Muramoto, K. Rice bran protein-based edible films. Int. J. Food Sci. Technol 2008, 43, 476–483. [Google Scholar]
- Ibrahim, N.A.; Ahmad, S.N.A.; Wan Md. Zin, W.Y.; Dahlan, K.Z.M. Effect of electron beam irradiation and poly(vinyl pyrrolidone) addition on mechanical properties of polycaprolactone with empty fruit bunch fibre (OPEFB) composite. eXPRESS Polym. Lett 2009, 3, 226–234. [Google Scholar]
- Park, S.B.; Hwang, S.Y.; Moon, C.W.; Im, S.S. Plasticizer effect of novel PBS ionomer in PLA/PBS ionomer blends. Macromol. Res 2010, 18, 463–471. [Google Scholar]
- Xu, H.; Wang, L.; Teng, C.; Yu, M. Biodegradable composites: Ramie fibre reinforced PLLA-PCL composite prepared by in situ polymerization process. Polym. Bull 2008, 61, 663–670. [Google Scholar]
- Zhang, N.; Wang, Q.; Ren, J.; Wang, L. Preparation and properties of biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blend with glycidyl methacrylate as reactive processing agent. J. Mater. Sci 2009, 44, 250–256. [Google Scholar]
- Todo, M.; Takayama, T. Improvement of mechanical properties of Poly(L-lactic acid) by blending of lysine triisocyanate. J. Mater. Sci 2007, 42, 4712–4715. [Google Scholar]
- Fenollar, O.; García, D.; Sánchez, L.; López, J.; Balart, R. Optimization of the curing conditions of PVC plastisols based on the use of an epoxidized fatty acid ester plasticizer. Eur. Polym. J 2009, 45, 2674–2684. [Google Scholar]
- Kim, H.B.; Choi, J.S.; Lee, C.H.; Lim, S.T.; Jhon, M.S.; Choi, H.J. Polymer blend/organoclay nanocomposite with poly(ethylene oxide) and poly(methyl methacrylate). Eur. Polym. J 2005, 41, 679–685. [Google Scholar]
- Liu, X.; Dever, M.; Fair, N.; Benson, R.S. Thermal and mechanical properties of poly(lactic acid) and poly(ethylene/butylene succinate) blends. J. Environ. Polym. Degrad 1997, 5, 225–235. [Google Scholar]
- Ibrahim, N.A.; Chieng, B.W.; Wan Md. Zin, W.Y. Morphology, thermal and mechanical properties of biodegradable poly(butylene succinate)/poly(butylene adipate-co-terephthalate)/clay nanocomposites. Polym. Plast. Technol. Eng 2010, 49, 1571–1580. [Google Scholar]
- Zhichao, L.; Pengju, P.; Bo, Z.; Tungalag, D.; Yoshio, I. Mechanical and thermal properties of poly(butylene succinate)/plant fiber biodegradable composite. J. Appl. Polym. Sci 2010, 115, 3559–3567. [Google Scholar]
- Seydibeyoglu, M.O.; Misra, M.; Mohanty, A. Synergistic improvements in the impact strength and % elongation of polyhydroxybutyrate-co-valerate copolymers with functionalized soybean oils and POSS. Int. J. Plast. Technol 2010, 14, 1–16. [Google Scholar]
- Chieng, B.W.; Ibrahim, N.A.; Wan Md. Zin, W.Y. Effect of organo-modified montmorillonite on poly(butylene succinate)/poly(butylene adipate-co-terephthalate) nanocomposites. eXPRESS Polym. Lett 2010, 4, 404–414. [Google Scholar]
- Emad, A.J.M. Preparation of new polymer nanocomposites based on poly(lactic acid)/fatty nitrogen compounds modified clay by a solution casting process. Fibers Polym 2011, 12, 444–450. [Google Scholar]
- Hala, F.N.; Mohamed, S.A.A.; Sherif, M.S.; Gamal, R.S. Synthesis and thermal characterization of poly(ester-ether urethane)s based on PHB and PCL-PEG-PCL blocks. J. Polym. Res 2011, 18, 1217–1227. [Google Scholar]
- Grishchuk, S.; Karger-Kocsis, J. Hybrid thermosets from vinyl ester resin and acrylated epoxidized soybean oil (AESO). eXPRESS Polym. Lett 2011, 5, 2–11. [Google Scholar]
- Jacob, J.; Ramasamy, M.; Mrinal, B. Evaluation of compatibility and properties of biodegradable polyester blends. J. Polym. Sci. Part A 2002, 40, 2003–2014. [Google Scholar]
- Joseph, C.S.; Prashanth, K.V.H.; Rastogi, N.K.; Indiramma, A.R.; Reddy, S.Y.; Raghavarao, K.S.M.S. Optimum blend of chitosan and poly-(ɛ-caprolactone) for fabrication of films for food packaging applications. Food Bioprocess Technol 2009, 4, 1179–1185. [Google Scholar]
- Boonfaung, P.; Wasutchanon, P.; Somwangthanaroj, A. Development of Packaging Film from Bioplastic Polylactic Acid (PLA) with Plasticizers. Proceedings of the Pure and Applied Chemistry International Conference 2011 (PACCON2011), Bangkok, Thailand, 5–7 January 2011.
- Katsuyoshi, S.; Shigetaka, F. The Effects of Plasticizer on the Mechanical and Electrical Characteristics of PLA. Proceedings of the 2008 International Symposium on Electrical Insulating Materials, Yokkaichi, Japan, 7–11 September 2008.
- Mitsuhiro, S.; Yoshihiro, S.; Masato, O.; Masanao, M. Thermal and mechanical properties of plasticized poly(L-lactide) nanocomposites with organo-modified montmorillonites. J. Appl. Polym. Sci 2006, 99, 2594–2602. [Google Scholar]
- Wang, Z.; Hou, X.; Mao, Z.; Ye, R.; Mo, Y.; Finlow, D.E. Synthesis and characterization of biodegradable poly(lactic acid-co-glycine) via direct melt copolymerization. Iran. Polym. J 2008, 17, 791–798. [Google Scholar]












| Sample Name | EPO(1) | EPO(2) | EPO(3) |
|---|---|---|---|
| Sample Composition | Epoxidized palm olein | Epoxidized palm kernel oil with small amount of soybean oil | Epoxidized palm olein with small amount of soybean oil |
| Oxygen Oxirane Content (%) | 3.2309 | 1.750 | 3.5803 |
| Acid Value (mg KOH/g sample) | 0.4287 | 0.1737 | 0.5990 |
| Iodine Value (g I2/100 g sample) | 0.6371 | 3.5674 | 0.4999 |
| Moisture Content | 0.08 | 0.03 | 0.06 |
| pH (pH test paper) | 5–6 | 5–6 | 5–6 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silverajah, V.S.G.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hassan, H.A.; Woei, C.B. A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend. Int. J. Mol. Sci. 2012, 13, 5878-5898. https://doi.org/10.3390/ijms13055878
Silverajah VSG, Ibrahim NA, Yunus WMZW, Hassan HA, Woei CB. A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend. International Journal of Molecular Sciences. 2012; 13(5):5878-5898. https://doi.org/10.3390/ijms13055878
Chicago/Turabian StyleSilverajah, V. S. Giita, Nor Azowa Ibrahim, Wan Md Zin Wan Yunus, Hazimah Abu Hassan, and Chieng Buong Woei. 2012. "A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend" International Journal of Molecular Sciences 13, no. 5: 5878-5898. https://doi.org/10.3390/ijms13055878