Neuroprotective Effects of Ischemic Preconditioning and Postconditioning on Global Brain Ischemia in Rats through the Same Effect on Inhibition of Apoptosis
Abstract
:1. Introduction
2. Results and Discussion
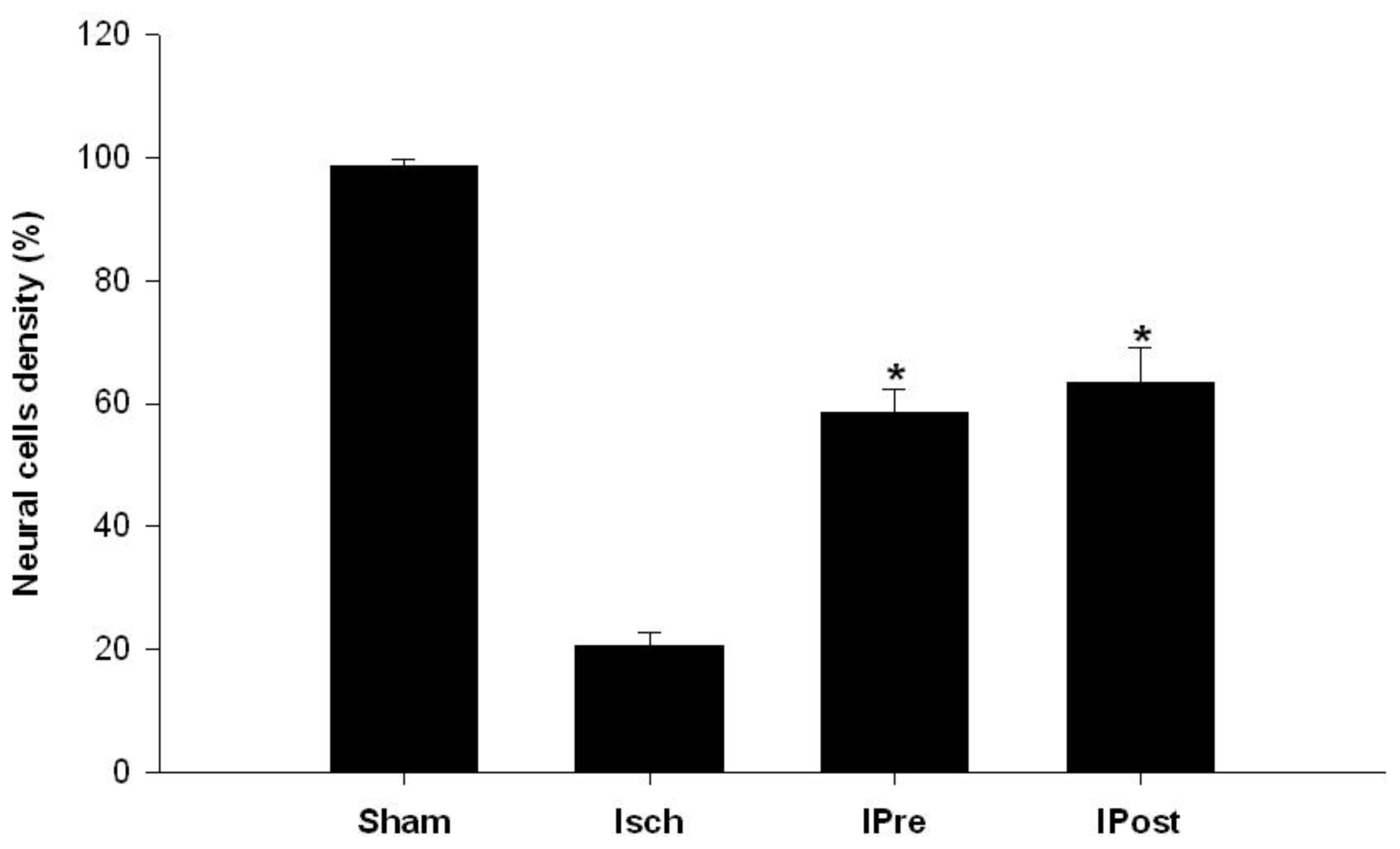
2.1. Neuronal Injury Following Global Cerebral Ischemia Is Reduced by Ischemic Preconditioning and Postconditioning
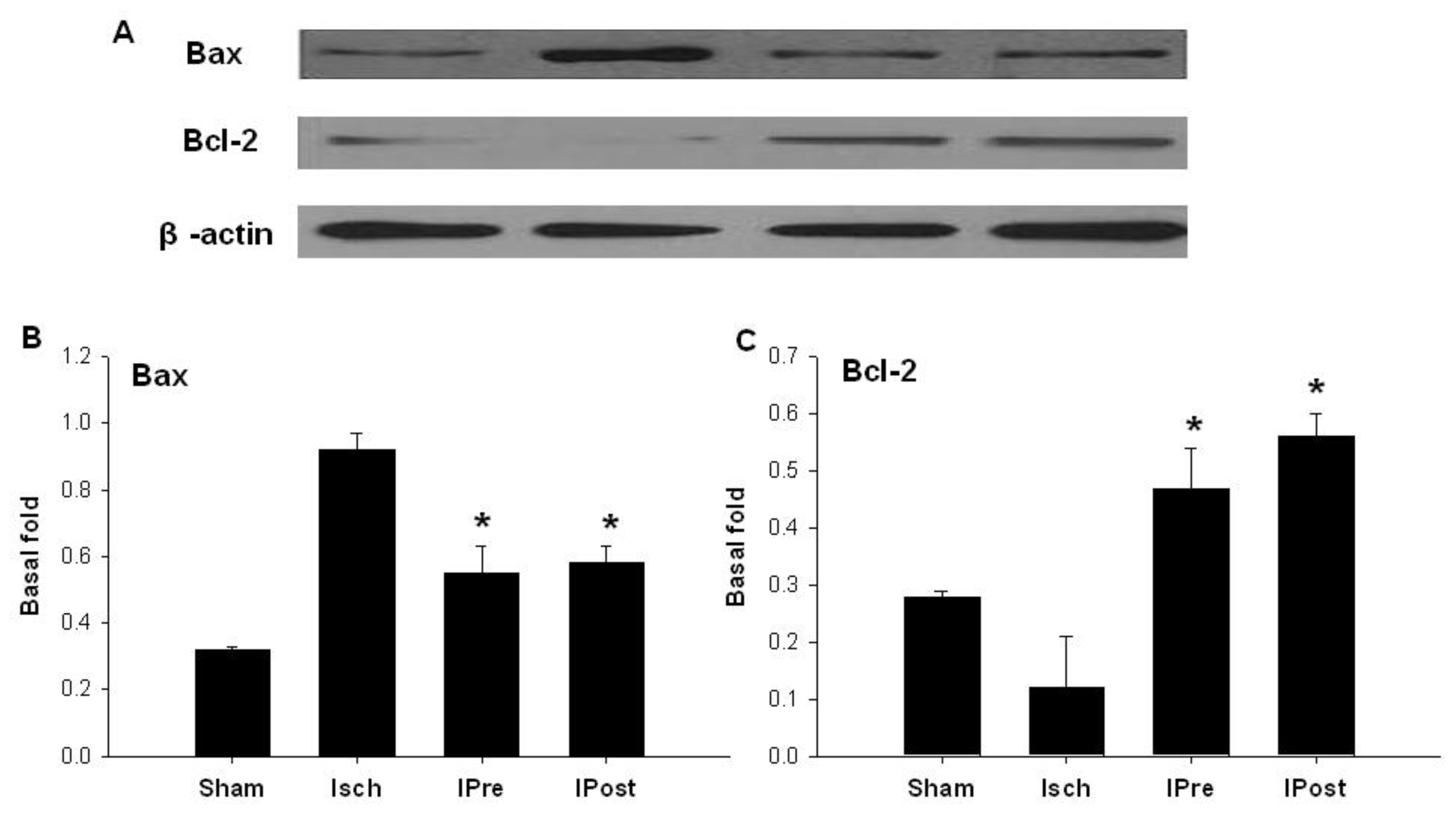
2.2. Downregulation of Hippocampal Cleaved Caspase-3, Cleaved Caspase-6, Cleaved Caspase-9 and Bax
2.3. Upregulation of Hippocampal Bcl-2
2.4. Discussion
3. Experimental Section
3.1. Animals
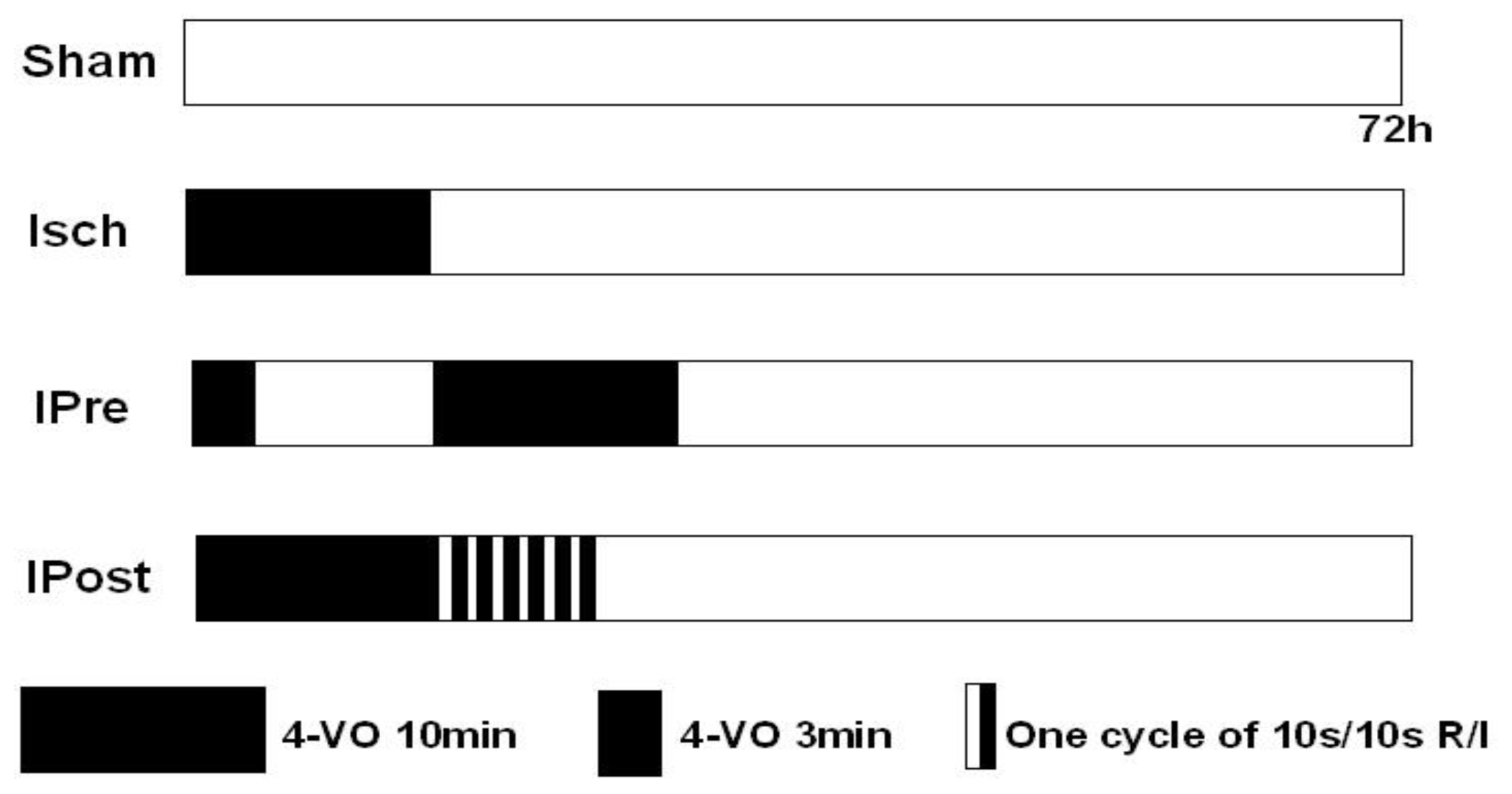
3.2. Establishment of the 4-Vessel Occlusion Model
3.3. Ischemic Preconditioning and Postconditioning
3.4. Histological Analysis
3.5. In Situ Labeling of DNA Fragmentation by TUNEL
3.6. Western Blotting
3.7. Statistical Analysis
4. Conclusions
Acknowledgement
- Conflict of InterestsThe author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Wardlaw, J.M.; von Kummer, R.; Farrall, A.J.; Chappell, F.M.; Hill, M.; Perry, D. A large web-based observer reliability study of early ischaemic signs on computed tomography. The Acute Cerebral CT Evaluation of Stroke Study (ACCESS). PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Sinanovic, O. Neuropsychology of acute stroke. Psychiatr. Danubina 2010, 22, 278–281. [Google Scholar]
- Van Bel, F.; Groenendaal, F. Long-term pharmacologic neuroprotection after birth asphyxia: Where do we stand? Neonatology 2008, 94, 203–210. [Google Scholar]
- Mantz, J.; Degos, V.; Laigle, C. Recent advances in pharmacologic neuroprotection. Eur. J. Anaesth 2010, 27, 6–10. [Google Scholar]
- Chavez, J.C.; Hurko, O.; Barone, F.C.; Feuerstein, G.Z. Pharmacologic interventions for stroke: Looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke J. Cereb. Circ 2009, 40, e558–e563. [Google Scholar]
- Durukan, A.; Tatlisumak, T. Preconditioning-induced ischemic tolerance: A window into endogenous gearing for cerebroprotection. Exp. Transl. Stroke Med 2010, 2. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Kim, Y.J. Mechanisms and prospects of ischemic tolerance induced by cerebral preconditioning. Int. Neurourol. J 2010, 14, 203–212. [Google Scholar]
- Lehotsky, J.; Burda, J.; Danielisova, V.; Gottlieb, M.; Kaplan, P.; Saniova, B. Ischemic tolerance: The mechanisms of neuroprotective strategy. Anat. Rec. (Hoboken) 2009, 292, 2002–2012. [Google Scholar]
- Ferrara, A.; El Bejaoui, S.; Seyen, S.; Tirelli, E.; Plumier, J.-C. The usefulness of operant conditioning procedures to assess long-lasting deficits following transient focal ischemia in mice. Behav. Brain Res 2009, 205, 525–534. [Google Scholar]
- Stenzel-Poore, M.P.; Stevens, S.L.; King, J.S.; Simon, R.P. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: A speculative synthesis. Stroke J.Cereb. Circ 2007, 38, 680–685. [Google Scholar]
- Miao, Y.; Zhang, W.; Lin, Y.; Lu, X.; Qiu, Y. Neuroprotective effects of ischemic preconditioning on global brain ischemia through up-regulation of acid-sensing ion channel 2a. Int. J. Mol. Sci 2010, 11, 140–153. [Google Scholar]
- Xing, B.; Chen, H.; Zhang, M.; Zhao, D.; Jiang, R.; Liu, X.; Zhang, S. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke J. Cereb. Circ 2008, 39, 2362–2369. [Google Scholar]
- Hausenloy, D.J.; Yellon, D.M. Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis 2009, 204, 334–341. [Google Scholar]
- Pignataro, G.; Scorziello, A.; di Renzo, G.; Annunziato, L. Post-ischemic brain damage: Effect of ischemic preconditioning and postconditioning and identification of potential candidates for stroke therapy. FEBS J 2009, 276, 46–57. [Google Scholar]
- Durukan, A.; Tatlisumak, T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Behav 2007, 87, 179–197. [Google Scholar]
- Plum, F. Neuroprotection in acute ischemic stroke. J. Am. Med. Assoc 2001, 285, 1760–1761. [Google Scholar]
- Ginsberg, M.D. Current status of neuroprotection for cerebral ischemia. Synoptic overview. Stroke 2009, 40, 111–114. [Google Scholar]
- Gladstone, D.J.; Black, S.E.; Hakim, A.M. Toward wisdom from failure-Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 2002, 33, 2123–2136. [Google Scholar]
- Kirino, T. Ischemic tolerance. J. Cereb. Blood Flow Metab 2002, 22, 1283–1296. [Google Scholar]
- Hausenloy, D.J.; Yellon, D.M. The therapeutic potential of ischemic conditioning: An update. Nat. Rev. Cardiol 2011, 8, 619–629. [Google Scholar]
- Kardesoglu, E.; Isilak, Z.; Uz, O.; Yiginer, O. Ischemic conditioning: A current concept in reducing reperfusion injury. Chin. Med. J 2011, 124, 480. [Google Scholar]
- Zhang, W.; Miao, Y.; Zhou, S.; Jiang, J.; Luo, Q.; Qiu, Y. Neuroprotective effects of ischemic postconditioning on global brain ischemia in rats through upregulation of hippocampal glutamine synthetase. J. Clin. Neurosci 2011, 18, 685–689. [Google Scholar]
- Kocher, A.A.; Schuster, M.D.; Szabolcs, M.J.; Takuma, S.; Burkhoff, D.; Wang, J.; Homma, S.; Edwards, N.M.; Itescu, S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med 2001, 7, 430–436. [Google Scholar]
- Vohra, H.A.; Galinanes, M. Effect of the degree of ischaemic injury and reoxygenation time on the type of myocardial cell death in man: Role of caspases. BMC Physiol 2005, 5. [Google Scholar] [CrossRef] [Green Version]
- Prunell, G.F.; Arboleda, V.A.; Troy, C.M. Caspase function in neuronal death: Delineation of the role of caspases in ischemia. CNS Neurol. Disord. Curr. Drug Target 2005, 4, 51–61. [Google Scholar]
- Yang, X.-Y.; Liu, Q.-N.; Zhang, L.; Jiang, S.-Q.; Gong, P.-L. Neuroprotective effect of dauricine after transient middle cerebral artery occlusion in rats: Involvement of Bcl-2 family proteins. Am. J. Chin. Med 2010, 38, 307–318. [Google Scholar]
- Koubi, D.; Jiang, H.; Zhang, L.; Tang, W.; Kuo, J.; Rodriguez, A.I.; Hunter, T.J.; Seidman, M.D.; Corcoran, G.B.; Levine, R.A. Role of Bcl-2 family of proteins in mediating apoptotic death of PC12 cells exposed to oxygen and glucose deprivation. Neurochem. Int 2005, 46, 73–81. [Google Scholar]
- Lim, S.Y.; Hausenloy, D.J. Remote ischemic conditioning: From bench to bedside. Front. Physiol 2012, 3. [Google Scholar] [CrossRef]
- Thielmann, M. Remote ischemic preconditioning in cardiac surgery: Caught between clinical relevance and statistical significance? Basic Res. Cardiol 2012, 107, 1–4. [Google Scholar]
- Yagi, T.; Yoshioka, H.; Wakai, T.; Kato, T.; Horikoshi, T.; Kinouchi, H. Activation of signal transducers and activators of transcription 3 in the hippocampal CA1 region in a rat model of global cerebral ischemic preconditioning. Brain Res 2011, 1422, 39–45. [Google Scholar]
- Hossmann, K.A. Cerebral ischemia: Models, methods and outcomes. Neuropharmacology 2008, 55, 257–270. [Google Scholar]




© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ding, Z.-M.; Wu, B.; Zhang, W.-Q.; Lu, X.-J.; Lin, Y.-C.; Geng, Y.-J.; Miao, Y.-F. Neuroprotective Effects of Ischemic Preconditioning and Postconditioning on Global Brain Ischemia in Rats through the Same Effect on Inhibition of Apoptosis. Int. J. Mol. Sci. 2012, 13, 6089-6101. https://doi.org/10.3390/ijms13056089
Ding Z-M, Wu B, Zhang W-Q, Lu X-J, Lin Y-C, Geng Y-J, Miao Y-F. Neuroprotective Effects of Ischemic Preconditioning and Postconditioning on Global Brain Ischemia in Rats through the Same Effect on Inhibition of Apoptosis. International Journal of Molecular Sciences. 2012; 13(5):6089-6101. https://doi.org/10.3390/ijms13056089
Chicago/Turabian StyleDing, Zhe-Min, Bing Wu, Wei-Qiao Zhang, Xiao-Jie Lu, Yu-Chang Lin, Yong-Jian Geng, and Yi-Feng Miao. 2012. "Neuroprotective Effects of Ischemic Preconditioning and Postconditioning on Global Brain Ischemia in Rats through the Same Effect on Inhibition of Apoptosis" International Journal of Molecular Sciences 13, no. 5: 6089-6101. https://doi.org/10.3390/ijms13056089



