Herbicide-Intercalated Zinc Layered Hydroxide Nanohybrid for a Dual-Guest Controlled Release Formulation
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of ZLH Nanohybrid
2.2. Herbicides Release Study
2.3. Characterization
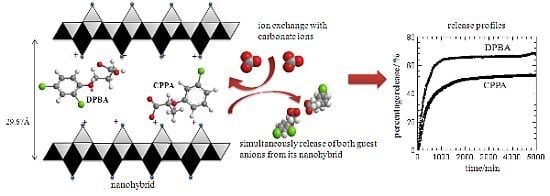
3. Results and Discussion
3.1. PXRD Analysis
3.2. Surface Morphology
3.3. Spatial Orientation of the Dual Herbicides in the ZLH Interlayers
3.4. Thermal Studies
3.5. FTIR Analysis
3.6. Herbicides Release Properties
4. Conclusions
Acknowledgment
References
- Miao, J.; Xue, M.; Itoh, H.; Feng, Q. Hydrothermal synthesis of layered hydroxide zinc benzoate compounds and their exfoliation reactions. J. Mater. Chem 2006, 16, 474–480. [Google Scholar]
- Lv, L.; Sun, P.; Gu, Z.; Du, H.; Pang, X.; Tao, X.; Xu, R.; Xu, L. Removal of chloride ion from aqueous solution by ZnAl-NO3 layered double hydroxides as anion-exchanger. J. Hazmat 2009, 161, 1444–1449. [Google Scholar]
- Nalawade, P.; Aware, B.; Kadam, V.J.; Hirlekar, R.S. Layered double hydroxides: A review. J. Sci. Ind. Res 2009, 68, 267–272. [Google Scholar]
- Kasai, A.; Fujihara, S. Layered single-metal hydroxide/ethylene glycol as a new class of hybrid material. Inorg. Chem 2006, 45, 415–418. [Google Scholar]
- Newman, S.P.; Jones, W. Comparative study of some layered hydroxide salts containing exchangeable interlayer anions. J. Solid State Chem 1999, 148, 26–40. [Google Scholar]
- Morioka, H.; Tagaya, H.; Kasaru, M.; Kadokawa, J.; Chiba, K. Effects of zinc on the new preparation method of hydroxyl double salts. Inorg. Chem 1999, 38, 4211–4216. [Google Scholar]
- Kandare, E.; Hossenlopp, J.M. Hydroxyl double salt anion exchange kinetics: Effects of precursor structure and anion size. J. Phys. Chem 2005, 109, 8469–8475. [Google Scholar]
- Hussein, M.Z.; Ghotbi, M.Y.; Yahaya, A.H.; Rahman, M.Z.A. Synthesis and characterization of (zinc-layered-gallate) nanohybrid using structural memory effect. Mater. Chem. Phys 2009, 113, 491–496. [Google Scholar]
- Marangoni, R.; Ramos, L.P.; Wypych, F. New multifunctional materials obtained by the intercalation of anionic dyes into layered zinc hydroxide nitrate followed by dispersion into Poly (vinyl alcohol) (PVA). J. Colloid Interface Sci 2009, 330, 303–309. [Google Scholar]
- Altuntasoglu, O.; Matsuda, Y.; Ida, S.; Matsumoto, Y. Syntheses of zinc oxide and zinc hydroxide single. Nanosheets Chem. Mater 2010, 22, 3158–3164. [Google Scholar]
- Zhao, L.; Miao, J.; Wang, H.; Ishikawa, Y.; Feng, Q. Synthesis and exfoliation of layered hydroxide zinc aminobenzoate compounds. J. Ceram. Soc. Jpn 2009, 117, 1115–1119. [Google Scholar]
- Arizaga, G.G.C.; Satyanarayana, K.G.; Wypych, F. Layered hydroxide salts: Synthesis, properties and potential applications. Solid State Ion 2007, 178, 1143–1162. [Google Scholar]
- Kuk, W.K.; Huh, Y.D. Preferential intercalation of organic anions into layered double hydroxide. Bull. Korean Chem. Soc 1998, 19, 1032–1036. [Google Scholar]
- Hwang, S.H.; Han, Y.S.; Choy, J.H. Intercalation of functional organic molecules with pharmaceutical, cosmeceutical and nutraceutical functions into layered double hydroxides and zinc basic salts. Bull. Korean Chem. Soc 2001, 22, 1019–1022. [Google Scholar]
- Yang, J.H.; Han, Y.S.; Park, M.; Park, T.; Hwang, S.J.; Choy, J.H. New inorganic-based drug delivery system of indole-3-acetic acid-layered metal hydroxide nanohybrids with controlled release rate. Chem. Mater 2007, 19, 2679–2685. [Google Scholar]
- Rocca, E.; Caillet, C.; Mesbah, A.; Francois, M.; Steinmetz, J. Intercalation in zinc-layered hydroxide: Zinc ydroxyheptanoate used as protective material on zinc. Chem. Mater 2006, 18, 6186–6193. [Google Scholar]
- Demel, J.; Kubát, P.; Jirka, I.; Kovář, P.; Pospíšil, M.; Lang, K. J. Phys. Chem 2010, 114, 16321–16328.
- Bull, R.M.R.; Markland, C.; Williams, G.R.; O’Hare, D. Hydroxy double salts as versatile storage and delivery matrices. J. Mater. Chem 2011, 21, 1822–1828. [Google Scholar]
- Hussein, M.Z.; Al Ali, S.H.; Zainal, Z.; Hakim, M.N. Development of antiproliferative nanohybrid compound with controlled release property using ellagic acid as the active agent. Int. J. Nanomed 2011, 6, 1373–1383. [Google Scholar]
- Cardoso, L.P.; Celis, R.; Cornejo, J.; Valim, J.B. Layered double hydroxides as supports for the sow release of acid herbicides. J. Agric. Food Chem 2006, 54, 5968–5975. [Google Scholar]
- Lewis, D.; Cowsar, D. Principles of Controlled Release Pesticides. In Controlled Release Pesticides; Scher, H.B., Ed.; Acs Symposium Series 57; American Chemical Society: Washington, DC, USA, 1977; pp. 1–14. [Google Scholar]
- Li, B.; He, J.; Evans, G.; Duan, X. Inorganic layered double hydroxides as a drug delivery system-intercalation and in vitro release of fenbufen. Appl. Clay Sci 2004, 27, 199–207. [Google Scholar]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar]
- Khan, A.I.; Ragavan, A.; Fong, B.; Markland, C.; O’Brien, M.; Dunbar, T.G.; Williams, G.R.; O’hare, D. Recent developments in the used of layered double hydroxides as host materials for the storage and triggered release of functional anions. Ind. Eng. Chem. Res 2009, 48, 10196–10205. [Google Scholar]
- Gasser, M.S. Inorganic layered double hydroxides as ascorbic acid (vitamin c) delivery system—Intercalation and their controlled release properties. Colloid Surf 2009, 73, 103–109. [Google Scholar]
- Sarijo, S.H.; Hussein, M.Z.; Yahaya, A.H.; Zainal, Z.; Yarmo, M.A. Synthesis of phenoxyherbicides-intercalated layered double hydroxide nanohybrids and their controlled release property. Curr. Nanosci 2010, 6, 199–205. [Google Scholar]
- Hussein, M.Z.; Hashim, N.; Yahaya, A.H.; Zainal, Z. Synthesis and characterization of [4-(2,4-dichlorophenoxybutyrate)-zinc layered hydroxide] nanohybrid. Solid State Sci 2010, 12, 770–775. [Google Scholar]
- Hussein, M.Z.; Rahman, N.S.S.A.; Sarijo, S.H.; Zainal, Z. Synthesis of a monophasic nanohybrid for a controlled release formulation of two active agents simultaneously. Appl. Clay Sci 2012, 58, 60–66. [Google Scholar]
- Xingfu, Z.; Zhaolin, H.; Yiqun, F.; Su, C.; Weiping, D.; Nanping, X. Microspheric organization of multilayered ZnO nanosheets with hierarchically porous structures. J. Phys. Chem 2008, 112, 11722–11728. [Google Scholar]










| Models | Zeroth Order | First Order | Parabolic Diffusion | Pseudo-Second Order | |||
|---|---|---|---|---|---|---|---|
| Anion | r2 | r2 | t1/2 (0−3000 min) | k (×10−5) (Lmg−1min−1) | c | ||
| CPPA | 0.79 | 0.86 | 0.92 | 0.93 | 543 | 2.82 | 8.31 |
| DPBA | 0.64 | 0.74 | 0.82 | 0.98 | 286 | 4.51 | 3.69 |
| equations | |||||||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hussein, M.Z.; Abdul Rahman, N.S.S.; Sarijo, S.H.; Zainal, Z. Herbicide-Intercalated Zinc Layered Hydroxide Nanohybrid for a Dual-Guest Controlled Release Formulation. Int. J. Mol. Sci. 2012, 13, 7328-7342. https://doi.org/10.3390/ijms13067328
Hussein MZ, Abdul Rahman NSS, Sarijo SH, Zainal Z. Herbicide-Intercalated Zinc Layered Hydroxide Nanohybrid for a Dual-Guest Controlled Release Formulation. International Journal of Molecular Sciences. 2012; 13(6):7328-7342. https://doi.org/10.3390/ijms13067328
Chicago/Turabian StyleHussein, Mohd Zobir, Nor Shazlirah Shazlyn Abdul Rahman, Siti H. Sarijo, and Zulkarnain Zainal. 2012. "Herbicide-Intercalated Zinc Layered Hydroxide Nanohybrid for a Dual-Guest Controlled Release Formulation" International Journal of Molecular Sciences 13, no. 6: 7328-7342. https://doi.org/10.3390/ijms13067328