Carrier Injection and Transport in Blue Phosphorescent Organic Light-Emitting Device with Oxadiazole Host
Abstract
:1. Introduction
2. Results and Discussion
2.1. Device Performances of Blue Phosphorescent OLEDs with Different Dopant Concentrations
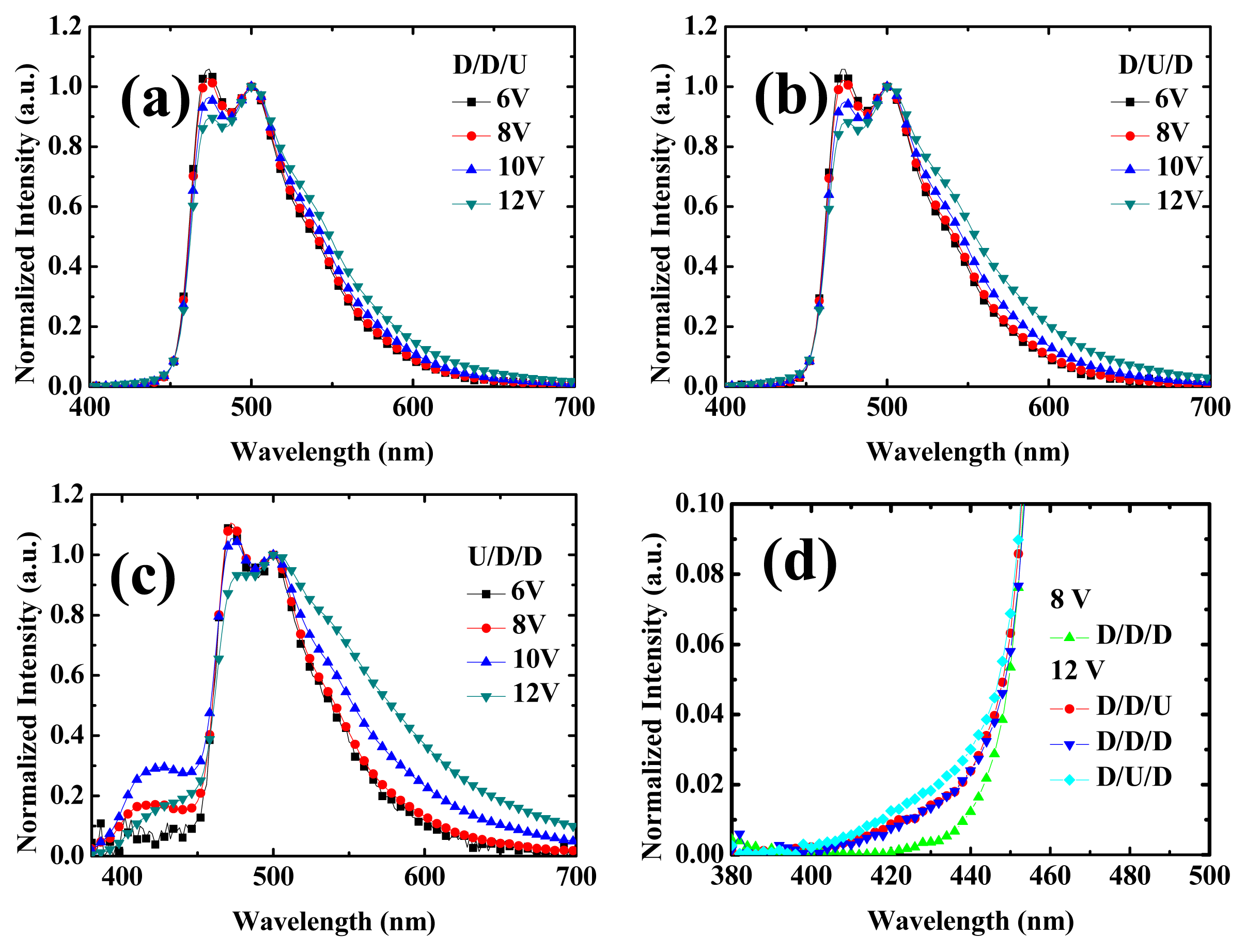
2.2. Device Performances of Blue Phosphorescent OLEDs with Different Doping Positions
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett 1987, 51, 913–915. [Google Scholar]
- Zhang, B.; Tan, G.; Lam, C.S.; Yao, B.; Ho, C.L.; Liu, L.; Xie, Z.; Wong, W.Y.; Ding, J.; Wang, L. High-efficiency single emissive layer white organic light-emitting diodes based on solution-processed dendritic host and new orange-emitting Iridium complex. Adv. Mater 2012, 24, 1873–1877. [Google Scholar]
- Zhou, G.; Wang, Q.; Ho, C.L.; Wong, W.Y.; Ma, D.; Wang, L. Duplicating “sunlight” from simple WOLEDs for lighting applications. Chem. Commun 2009, 3574–3576. [Google Scholar]
- Zhou, G.; Wong, W.Y.; Suo, S. Recent progress and current challenges in phosphorescent white organic light-emitting diodes (WOLEDs). J. Photochem. Photobiol. C Photochem. Rev 2010, 11, 133–156. [Google Scholar]
- Wu, H.; Zhou, G.J.; Zou, J.; Ho, C.L.; Wong, W.Y.; Yang, W.; Peng, J.; Cao, Y. Efficient polymer white-light-emitting devices for solid-state lighting. Adv. Mater 2009, 21, 4181–4184. [Google Scholar]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lüssem, B.; Leo, K. White organic light-emitting diodes with fluorescent tube efficiency. Nature 2009, 459, 234–238. [Google Scholar]
- Zheng, T.; Choy, W.C.H. High efficiency blue organic LEDs achieved by an integrated fluorescence–interlayer–phosphorescence emission architecture. Adv. Funct. Mater 2010, 20, 648–655. [Google Scholar]
- Chou, H.H.; Cheng, C.H. A highly efficient universal bipolar host for blue, green, and red phosphorescent OLEDs. Adv. Mater 2010, 22, 2468–2471. [Google Scholar]
- Fan, C.; Chen, Y.; Jiang, Z.; Yang, C.; Zhong, C.; Qin, J.; Ma, D. Diarylmethylene-bridged triphenylamine derivatives encapsulated with fluorene: Very high Tg host materials for efficient blue and green phosphorescent OLEDs. J. Mater. Chem 2010, 20, 3232–3237. [Google Scholar]
- Jang, S.E.; Joo, C.W.; Jeon, S.O.; Yook, K.S.; Lee, J.Y. The relationship between the substitution position of the diphenylphosphine oxide on the spirobifluorene and device performances of blue phosphorescent organic light-emitting diodes. Org. Electron 2010, 11, 1059–1065. [Google Scholar]
- Lee, J.; Lee, J.I.; Chu, H.Y. Improved performance of white phosphorescent organic light-emitting diodes through a mixed-host structure. ETRI J 2009, 31, 642–646. [Google Scholar]
- Kappaun, S.; Slugovc, C.; List, E.J.W. Phosphorescent organic light-emitting devices: Working principle and iridium based emitter materials. Int. J. Mol. Sci 2008, 9, 1527–1547. [Google Scholar]
- Cheng, G.; Zhang, Y.; Zhao, Y.; Lin, Y.; Ruan, C.; Liu, S.; Fei, T.; Ma, Y.; Cheng, Y. White organic light-emitting devices with a phosphorescent multiple emissive layer. Appl. Phys. Lett 2006, 89, 043504:1–043504:3. [Google Scholar]
- Zhou, G.; Wong, W.Y.; Yang, X. New design tactics in OLEDs using functionalized 2-phenylpyridine-type cyclometalates of Iridium(III) and Platinum(II). Chem. Asian J 2011, 6, 1706–1727. [Google Scholar]
- Wong, W.Y.; Ho, C.L. Heavy metal organometallic electrophosphors derived from multi-component chromophores. Coord. Chem. Rev 2009, 253, 1709–1758. [Google Scholar]
- Lee, J.H.; Ho, Y.H.; Lin, T.C.; Wu, C.F. High-efficiency fluorescent blue organic light-emitting device with balanced carrier transport. J. Electrochem. Soc 2007, 154, J226–J228. [Google Scholar]
- Lee, J.H.; Huang, C.L.; Hsiao, C.H.; Leung, M.K.; Yang, C.C.; Chao, C.C. Blue phosphorescent organic light-emitting device with double emitting layer. Appl. Phys. Lett 2009, 94, 223301:1–223301:3. [Google Scholar]
- Lan, Y.H.; Hsiao, C.H.; Lee, P.Y.; Bai, Y.C.; Lee, C.C.; Yang, C.C.; Leung, M.K.; Wei, M.K.; Chiu, T.L.; Lee, J.H. Dopant effects in phosphorescent white organic light-emitting device with double-emitting layer. Org. Electron 2011, 12, 756–765. [Google Scholar]
- Zou, J.; Wu, H.; Lam, C.S.; Wang, C.; Zhu, J.; Zhong, C.; Hu, S.; Ho, C.L.; Zhou, G.J.; Wu, H.; et al. Simultaneous optimization of charge-carrier balance and luminous efficacy in highly efficient white polymer light-emitting devices. Adv. Mater 2011, 23, 2976–7980. [Google Scholar]
- Wong, W.Y.; Ho, C.L. Functional metallophosphors for effective charge carrier injection/transport: New robust OLED materials with emerging applications. J. Mater. Chem 2009, 19, 4457–4482. [Google Scholar]
- Choi, W.H.; Cheung, C.H.; So, S.K. Can an organic phosphorescent dye act as a charge transporter? Org. Electron 2010, 11, 872–875. [Google Scholar]
- Adachi, C.; Kwong, R.; Forrest, S.R. Efficient electrophosphorescence using a doped ambipolar conductive molecular organic thin film. Org. Electron 2001, 2, 37–43. [Google Scholar]
- Matsusue, N.; Ikame, S.; Suzuki, Y.; Naito, H. Charge-carrier transport and triplet exciton diffusion in a blue electrophosphorescent emitting layer. J. Appl. Phys 2005, 97, 123512:1–123512:5. [Google Scholar]
- Matsusue, N.; Ikame, S.; Suzuki, Y.; Naito, H. Charge carrier transport in an emissive layer of green electrophosphorescent devices. Appl. Phys. Lett 2004, 85, 4046–4048. [Google Scholar]
- Noh, S.; Suman, C.K.; Hong, Y.; Lee, C. Carrier conduction mechanism for phosphorescent material doped organic semiconductor. J. Appl. Phys 2009, 105, 033709:1–033709:5. [Google Scholar]
- Chiu, T.L.; Lee, J.H.; Hsiao, Y.P.; Lin, C.F.; Chao, C.C.; Leung, M.K.; Wan, D.H.; Chen, H.L.; Ho, H.C. Absorptive and conductive cavity cathode with silver nanoparticles for low reflection organic light-emitted devices. J. Phys. D Appl. Phys 2011, 44, 095102:1–095102:6. [Google Scholar]
- Chiu, T.L.; Xu, W.F.; Lin, C.F.; Lee, J.H.; Chao, C.C.; Leung, M.K. Optical and electrical characteristics of Ag-doped perylene diimide derivative. Appl. Phys. Lett 2009, 94, 13307:1–13307:3. [Google Scholar]
- Li, X.C.; Yong, T.M.; Grüner, J.; Holmes, A.B.; Moratti, S.C.; Cacialli, F.; Friend, R.H. A blue light emitting copolymer with charge transporting and photo-crosslinkable functional units. Synth. Met 1997, 84, 437–438. [Google Scholar]
- Li, X.C.; Kraft, A.; Cervini, R.; Spencer, G.C.W.; Cacialli, F.; Friend, R.H.; Grüner, J.; Holmes, A.B.; DeMello, J.C.; Moratti, S.C. Synthesis and optoelectronic properties of oxadiazole-based polymers. Mater. Res. Soc. Symp. Proc 1996, 413, 13–22. [Google Scholar]
- He, Z.; Wong, W.Y.; Yu, X.; Kwok, H.S.; Lin, Z. Phosphorescent Platinum(II) complexes derived from multifunctional chromophores: Synthesis, structures, photophysics, and electroluminescence. Inorg. Chem 2006, 45, 10922–10937. [Google Scholar]
- Wong, W.Y.; He, Z.; So, S.K.; Tong, K.L.; Lin, Z. A multifunctional platinum-based triplet emitter for OLED applications. Organometallics 2005, 24, 4079–4082. [Google Scholar]
- Chiu, T.L.; Lee, P.Y.; Lee, J.H.; Hsiao, C.H.; Leung, M.K.; Lee, C.C.; Chen, C.Y.; Yang, C.C. Oxadiazole host for a phosphorescent organic light-emitting device. J. Appl. Phys 2011, 109, 084520:1–084520:17. [Google Scholar]
- Yu, X.M.; Zhou, G.J.; Lam, C.S.; Wong, W.Y.; Zhu, X.L.; Sun, J.X.; Wong, M.; Kwok, H.S. A yellow-emitting iridium complex for use in phosphorescent multiple-emissive-layer white organic light-emitting diodes with high color quality and efficiency. J. Organomet. Chem 2008, 693, 1518–1527. [Google Scholar]
- Yu, X.M.; Kwok, H.S.; Wong, W.Y.; Zhou, G.J. High-efficiency white organic light-emitting devices based on a highly amorphous Iridium(III) orange phosphor. Chem. Mater 2006, 18, 5097–5103. [Google Scholar]





| Device | HTL | EBL | EML (30 nm) | ETL | EIL | Cathode | ||
|---|---|---|---|---|---|---|---|---|
| NPB | mCP | FIrpic in OXD | BPhen | LiF | Al | |||
| 1 | 0% | |||||||
| 2 | 3% | |||||||
| 3 | 6% | |||||||
| 4 | 9% | |||||||
| 5 | 12% | |||||||
| 6 | 50 nm | 10 nm | 15% | 40 nm | 1.2 nm | 100 nm | ||
| Device | EML1 (10 nm) | EML2 (10 nm) | EML3 (10 nm) | |||||
| 7 | 9% | 9% | 0% | |||||
| 8 | 9% | 0% | 9% | |||||
| 9 | 0% | 9% | 9% | |||||
| Device | Volt. @5 mA/cm2 | Max. lm/W | Max. cd/A |
|---|---|---|---|
| 1 | 11.6 | 0.17 @ 5.5 V | 0.77 @ 5 V |
| 2 | 9.63 | 3.33 @ 6 V | 6.38 @ 6.5 V |
| 3 | 9.87 | 5.65 @ 6 V | 11.4 @ 6.5 V |
| 4 | 9.67 | 6.29 @ 6 V | 13 @ 6.5 V |
| 5 | 8.78 | 6.80 @ 6 V | 14.2 @ 7 V |
| 6 | 9.16 | 6.29 @ 6 V | 14.1 @ 7.5 V |
| 7 | 10.6 | 5.34 @ 6 V | 10.6 @ 6.5 V |
| 8 | 10 | 5.47 @ 6 V | 10.9 @ 6.5 V |
| 9 | 9.88 | 1.13 @ 6 V | 2.21 @ 7 V |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chiu, T.-L.; Lee, P.-Y. Carrier Injection and Transport in Blue Phosphorescent Organic Light-Emitting Device with Oxadiazole Host. Int. J. Mol. Sci. 2012, 13, 7575-7585. https://doi.org/10.3390/ijms13067575
Chiu T-L, Lee P-Y. Carrier Injection and Transport in Blue Phosphorescent Organic Light-Emitting Device with Oxadiazole Host. International Journal of Molecular Sciences. 2012; 13(6):7575-7585. https://doi.org/10.3390/ijms13067575
Chicago/Turabian StyleChiu, Tien-Lung, and Pei-Yu Lee. 2012. "Carrier Injection and Transport in Blue Phosphorescent Organic Light-Emitting Device with Oxadiazole Host" International Journal of Molecular Sciences 13, no. 6: 7575-7585. https://doi.org/10.3390/ijms13067575




