Chemopreventive Efficacy of Atorvastatin against Nitrosamine-Induced Rat Bladder Cancer: Antioxidant, Anti-Proliferative and Anti-Inflammatory Properties
Abstract
:1. Introduction
2. Results
2.1. Bladder Cancer Development and Growth
2.1.1. Macroscopic Evaluation
2.1.2. Quantitative Evaluation
2.1.3. Qualitative Evaluation
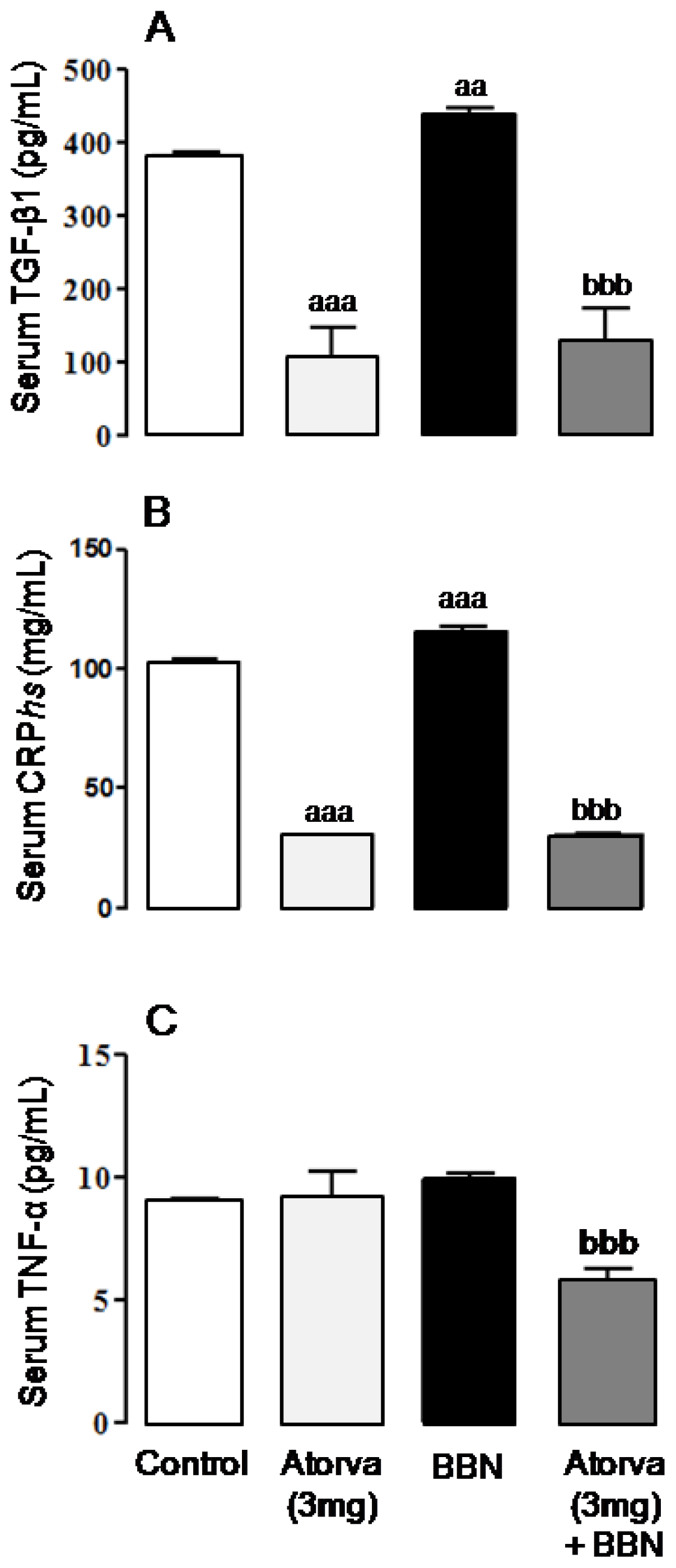
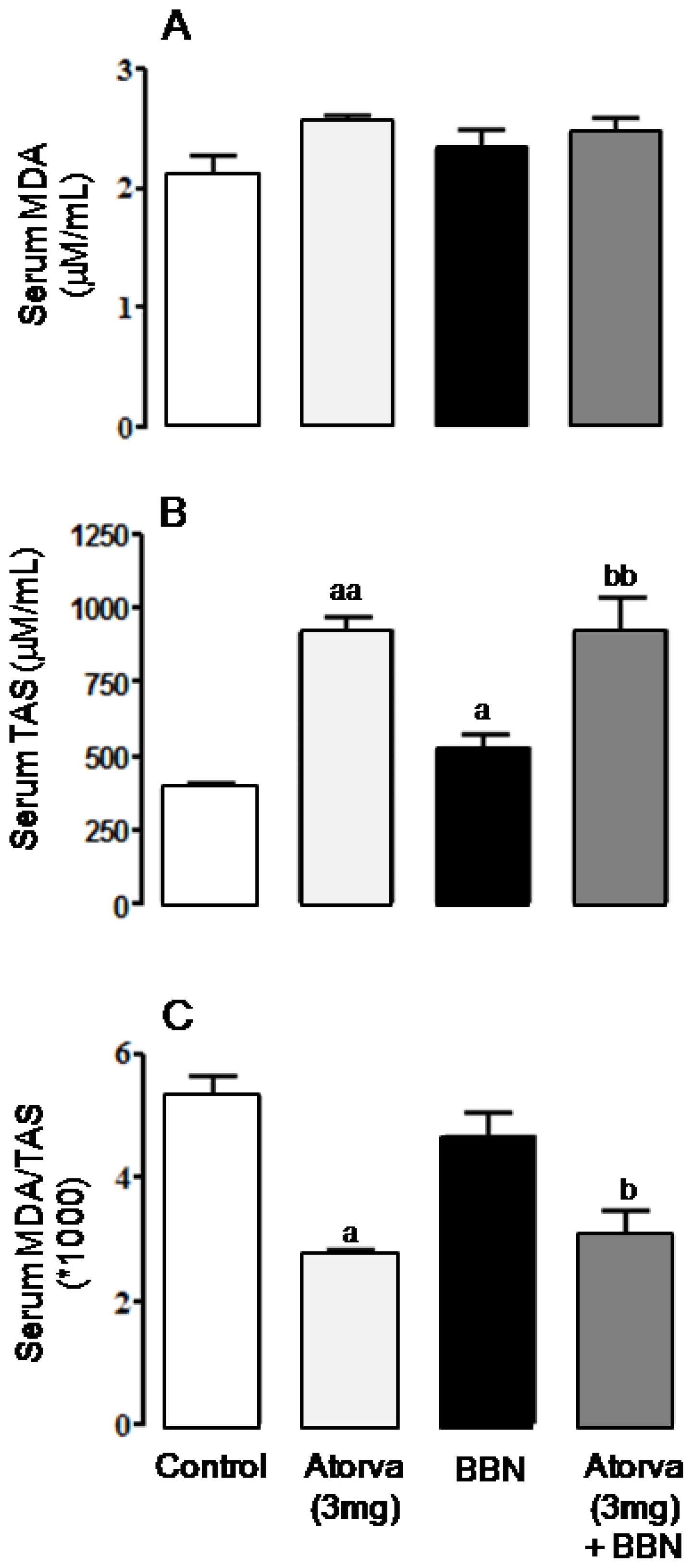
2.2. Systemic Proliferation, Inflammation and Oxidative Stress Markers
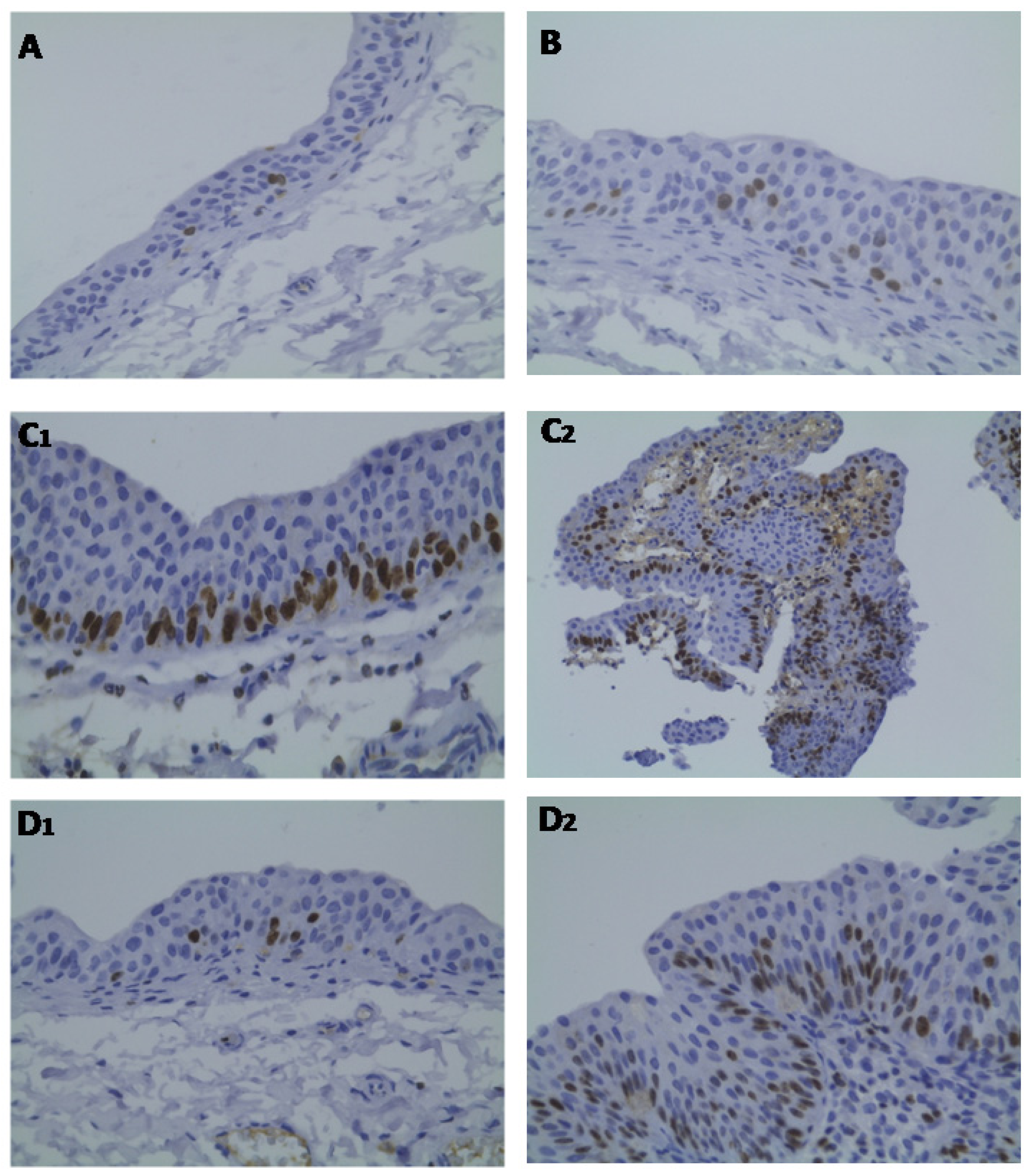
2.3. Bladder Cancer p53, ki67 and CD31 Immunohistochemistry
2.4. Biochemical Data: Safety Profile
3. Discussion
4. Experimental Section
4.1. Animals and Groups
4.2. Sample Collection and Preparation
4.3. Tumor Chemoprevention Analysis
4.3.1. Quantitative Analysis (Number and Tumor Volume)
4.3.2. Qualitative Analysis (Bladder Histology)
4.4. Proliferation, Inflammation and Redox Status Markers
4.5. Bladder Cancer p53, ki67 and CD31 Immunohistochemistry
4.6. Biochemical Assays (Safety Profile)
4.7. Statistical Analysis
5. Conclusions
References
- Grasso, M. Bladder cancer: A major public health issue. Eur. Urol. Suppl 2008, 7, 510–515. [Google Scholar]
- Ferlay, J.; Autier, P.; Boniol, M.; Heanue, M.; Colombet, M.; Boyle, P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann. Oncol 2007, 18, 581–592. [Google Scholar]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging, grading and diagnosis. Urology 2005, 66, 4–34. [Google Scholar]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol 2006, 49, 466–477. [Google Scholar]
- Malkowicz, S.B.; van Poppel, H.; Mickisch, G.; Pansadoro, V.; Thüroff, J.; Soloway, M.S.; Chang, S.; Benson, M.; Fukui, I. Muscle-invasive urothelial carcinoma of the bladder. Urology 2007, 69, 3–16. [Google Scholar]
- Zeegers, M.P.; Tan, F.E.; Dorant, E.; van den Brandt, P.A. The impact of characteristics of cigarette smoking on urinary tract cancer risk: A meta-analysis of epidemiologic studies. Cancer 2000, 66, 4–34. [Google Scholar]
- Kogevinas, M.; 't Mannetje, A.; Cordier, S.; Ranft, U.; González, C.A.; Vineis, P.; Chang-Claude, J.; Lynge, E.; Wahrendorf, J.; Tzonou, A.; et al. Occupation and bladder cancer among men in Western Europe. Cancer Causes Contr 2003, 14, 907–914. [Google Scholar]
- Wolff, D.J. The genetics of bladder cancer: A cytogeneticist’s perspective. Cytogenet. Genome Res 2007, 118, 177–181. [Google Scholar]
- Pelucchi, C.; La Vecchia, C. Alcohol, coffee, and bladder cancer risk: A review of epidemiological studies. Eur. J. Cancer Prev 2009, 18, 62–68. [Google Scholar]
- Kunze, E.; Schauer, A.; Schatt, S. Stages of transformation in the development of Nbutyltransitional cell carcinomas in the urinary bladder of rats. Z. Krebsforsch. Klin. Onkol 1976, 87, 139–160. [Google Scholar]
- Oliveira, P.A.; Colaço, A.; de la Cruz, L.F.; Lopes, C. Experimental bladder carcinogenesis-rodent models. Exp. Oncol 2006, 28, 2–11. [Google Scholar]
- Fukushimam, S.; Hirose, M.; Tsuda, H.; Shirai, T.; Hirao, K. Histological classification of urinary bladder cancers in rats induced by N-butyl-n-(4-hydroxybutyl) nitrosamine. Gann 1976, 67, 81–90. [Google Scholar]
- Hameed, D.A.; el-Metwally, T.H. The effectiveness of retinoic acid treatment in bladder cancer: Impact on recurrence, survival and TGF-alpha and VEGF as end-point biomarkers. Canc. Biol. Ther 2008, 7, 92–100. [Google Scholar]
- Parada, B.; Reis, F.; Figueiredo, A.; Nunes, P.; Teixeira-Lemos, E.; Garrido, P.; Sereno, J.; Pinto, R.; Cunha, M.F.; Neto, P.; et al. Inhibition of bladder tumor growth by sirolimus in an experimental carcinogenesis model. BJU Int 2011, 107, 135–143. [Google Scholar]
- Parada, B.; Sereno, J.; Reis, F.; Teixeira-Lemos, E.; Garrido, P.; Pinto, A.F.; Cunha, M.F.; Pinto, R.; Mota, A.; Figueiredo, A.; et al. Anti-inflammatory, anti-proliferative and antioxidant profiles of selective cyclooxygenase-2 inhibition as chemoprevention for rat bladder carcinogenesis. Cancer Biol.Ther 2009, 8, 1615–1622. [Google Scholar]
- Demierre, M.F.; Higgins, P.; Gruber, S.B.; Hawk, E.; Lippman, S.M. Statins and cancer prevention. Nature 2005, 5, 930–942. [Google Scholar]
- Brown, A.J. Cholesterol, statins and cancer. Clin. Exp. Pharmacol. Physiol 2007, 34, 135–141. [Google Scholar]
- Sassano, A.; Platanias, L.C. Statins in tumor suppression. Cancer Lett 2008, 260, 11–19. [Google Scholar]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Ann. Rev. Pharmacol. Toxicol 2005, 45, 89–111. [Google Scholar]
- Schöenbeck, U.; Libby, P. Inflammation, immunity, and HMG-CoA reductase inhibitors: Statins as anti-inflammatory agents? Circulation 2004, 109, 18–26. [Google Scholar]
- Wassmann, S.; Laufs, U.; Müller, K.; Konkol, C.; Ahlbory, K.; Bäumer, A.T.; Linz, W.; Böhm, M.; Nickenig, G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol 2002, 22, 300–305. [Google Scholar]
- Tanaka, K.; Honda, M.; Takabatake, T. Anti-apoptotic effect of atorvastatin, a 3-hydroxy-3- methylglutaryl coenzyme a reductase inhibitor, on cardiac myocytes through protein kinase C activation. Clin. Exp. Pharmacol. Physiol 2004, 31, 360–364. [Google Scholar]
- Dulak, J.; Józkowicz, A. Anti-angiogenic and anti-inflammatory effects of statins: Relevance to anti-cancer therapy. Curr. Cancer Drug Targets 2005, 5, 579–594. [Google Scholar]
- Kusama, T.; Mukai, M.; Iwasaki, T.; Tatsuta, M.; Matsumoto, Y.; Akedo, H.; Inoue, M.; Nakamura, H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology 2002, 122, 308–317. [Google Scholar]
- Hamilton, R.J.; Freedland, S.J. Rationale for statins in the chemoprevention of prostate cancer. Curr. Urol. Rep 2008, 9, 189–196. [Google Scholar]
- Calabro, A.; Tai, J.; Allen, S.L.; Budman, D.R. In vitro synergism of m-TOR inhibitors, statins, and classical chemotherapy: Potential implications in acute leukemia. Anti-cancer Drugs 2008, 19, 705–712. [Google Scholar]
- Schmidmaier, R.; Simsek, M.; Baumann, P.; Emmerich, B.; Meinhardt, G. Synergistic antimyeloma effects of zoledronate and simvastatin. Anti-cancer Drugs 2006, 17, 621–629. [Google Scholar]
- Kamat, A.M.; Nelkin, G.M. Atorvastatin: A potential chemopreventive agent in bladder cancer. Urology 2005, 66, 1209–1212. [Google Scholar]
- Kaye, J.A.; Jick, H. Statin use and cancer risk in the General Practice Research Database. Br. J . Cancer 2004, 90, 635–637. [Google Scholar]
- Kitayama, J.; Hatano, K.; Kaisaki, S.; Suzuki, H.; Fujii, S.; Nagawa, H. Hyperlipidaemia is positively correlated with lymph node metastasis in men with early gastric cancer. Br. J. Surg. 2004, 91, 191–198. [Google Scholar]
- Manickavasagam, S.; Ye, Y.; Lin, Y.; Perez-Polo, R.J.; Huang, M.H.; Lui, C.Y.; Hughes, M.G.; McAdoo, D.J.; Uretsky, B.F.; Birnbaum, Y. The cardioprotective effect of a statin and cilostazol combination: Relationship to Akt and endothelial nitric oxide synthase activation. Cardiovasc. Drugs. Ther 2007, 21, 321–330. [Google Scholar]
- Birnbaum, Y.; Lin, Y.; Ye, Y.; Merla, R.; Perez-Polo, J.R.; Uretsky, B.F. Pretreatment with high-dose statin, but not low-dose statin, ezetimibe, or the combination of low-dose statin and ezetimibe, limits infarct size in the rat. J. Cardiovasc. Pharmacol. Ther 2008, 13, 72–79. [Google Scholar]
- Schmechel, A.; Grimm, M.; El-Armouche, A.; Höppner, G.; Schwoerer, A.P.; Ehmke, H.; Eschenhagen, T. Treatment with atorvastatin partially protects the rat heart from harmful catecholamine effects. Cardiovasc. Res 2009, 82, 100–106. [Google Scholar]
- Wierzbicki, A.S.; Poston, R.; Ferro, A. The lipid and non-lipid effects of statins. Pharmacol. Ther 2003, 99, 95–112. [Google Scholar]
- Chan, K.K.W.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Canc. Res 2003, 9, 10–19. [Google Scholar]
- Bababeygy, S.R.; Polevaya, N.V.; Youssef, S.; Sun, A.; Xiong, A.; Prugpichailers, T.; Veeravagu, A.; Hou, L.C.; Steinman, L.; Tse, V. HMG-CoA reductase inhibition causes increased necrosis and apoptosis in an in vivo mouse glioblastoma multiforme model. Anticancer Res 2009, 29, 4901–4908. [Google Scholar]
- Jánosi, J.; Sebestyén, A.; Bocsi, J.; Barna, G.; Nagy, K.; Vályi-Nagy, I.; Kopper, L. Mevastatin-induced apoptosis and growth suppression in U266 myeloma cells. Anticancer Res 2004, 24, 1817–1822. [Google Scholar]
- Fujita, J.; Yoshida, O.; Yuasa, Y.; Rhim, J.S.; Hatanaka, M.; Aaronson, S. Ha-ras oncogenes are activated by somatic alterations in human urinary tract tumors. Nature 1984, 309, 464–466. [Google Scholar]
- Oxford, G.; Theodorescu, D. The role of Ras superfamily proteins in bladder cancer progression. J .Urol 2003, 170, 1987–1993. [Google Scholar]
- Björkhem-Bergman, L.; Acimovic, J.; Torndal, U.B.; Parini, P.; Eriksson, L.C. Lovastatin prevents carcinogenesis in a rat model for liver cancer. Effects of ubiquinone supplementation. Anticancer Res 2010, 30, 1105–1112. [Google Scholar]
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov 2005, 4, 977–987. [Google Scholar]
- Paumelle, R.; Staels, B. Peroxisome proliferators-activated receptors mediate pleiotropic actions of statins. Circ. Res 2007, 100, 1394–1395. [Google Scholar]
- Michalik, L.; Desvergne, B.; Wahli, W. Peroxisome proliferators-activated receptors and cancer: Complex stories. Nat. Rev 2004, 4, 61–70. [Google Scholar]
- Mansure, J.J.; Nassim, R.; Kassouf, W. Peroxisome proliferator-activated receptor gamma in bladder cancer: A promising therapeutic target. Cancer Biol. Ther 2009, 8, 6–15. [Google Scholar]
- Zheng, X.; Cui, X.X.; Avila, G.E.; Huang, M.T.; Liu, Y.; Patel, J.; Kong, A.N.; Paulino, R.; Shih, W.J.; Lin, Y.; et al. Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clin. Cancer Res 2007, 13, 5480–5487. [Google Scholar]
- Keller, J.J.; Giardiello, F.M. Chemoprevention strategies using NSAIDs and COX-2 inhibitors. Canc. Biol. Ther 2003, 2, S140–S149. [Google Scholar]
- El Gehani, K.; Al-Kikhia, L.; Mansuri, N.; Syrjänen, K.; Al-Fituri, O.; Elzagheid, A. Angiogenesis in urinary bladder carcinoma as defined by microvessel density (MVD) after immunohistochemical staining for Factor VIII and CD31. Libyan. J. Med. 2011, 6, 46. [Google Scholar] [CrossRef]
- Goebell, P.J.; Groshen, S.G.; Schmitz-Dräger, B.J. International Study-Initiative on Bladder Cancer (ISBC). p53 immunohistochemistry in bladder cancer- a new approach to an old question. Urol. Oncol 2010, 28, 377–388. [Google Scholar]
- Lara, P.C.; Rey, A.; Santana, C.; Afonso, J.L.; Diaz, J.M.; González, G.J.; Apolinario, R. The role of Ki67 proliferation assessment in predicting local control in bladder cancer patients treated by radical radiation therapy. Radiother. Oncol 1998, 49, 163–167. [Google Scholar]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar]
- Coyle, C.H.; Philips, B.J.; Morrisroe, S.N.; Chancellor, M.B.; Yoshimura, N. Antioxidant effects of green tea and its polyphenols on bladder cells. Life Sci 2008, 83, 12–18. [Google Scholar]
- Piloto, N.; Teixeira, H.M.; Teixeira-Lemos, E.; Parada, B.; Garrido, P.; Sereno, J.; Pinto, R.; Carvalho, L.; Costa, E.; Belo, L.; et al. Erythropoietin promotes deleterious cardiovascular effects and mortality risk in a rat model of chronic sports doping. Cardiovasc. Toxicol 2009, 9, 201–210. [Google Scholar]
- Kelsey, K.T.; Hirao, T.; Schned, A.; Hirao, S.; Devi-Ashok, T.; Nelson, H.H.; Andrew, A.; Karagas, M.R. A population-based study of immunohistochemical detection of p53 alteration in bladder cancer. Br. J. Cancer 2004, 90, 1572–1576. [Google Scholar]
- Lopez-Beltran, A.; Luque, R.J.; Alvarez-Kindelan, J.; Quintero, A.; Merlo, F.; Requena, M.J.; Montironi, R. Prognostic factors in survival of patients with stage Ta and T1 bladder urothelial tumors: The role of G1-S modulators (p53, p21Waf1, p27Kip1, cyclin D1, and cyclin D3), proliferation index, and clinicopathologic parameters. Am. J. Clin. Pathol 2004, 122, 444–452. [Google Scholar]
- Taylor, J.A., III; Kuchel, G.A.; Hegde, P.; Voznesensky, O.S.; Claffey, K.; Tsimikas, J.; Leng, L.; Bucala, R.; Pilbeam, C. Null mutation for macrophage migration inhibitory factor (MIF) is associated with less aggressive bladder cancer in mice. BMC Cancer 2007, 7, 135. [Google Scholar]
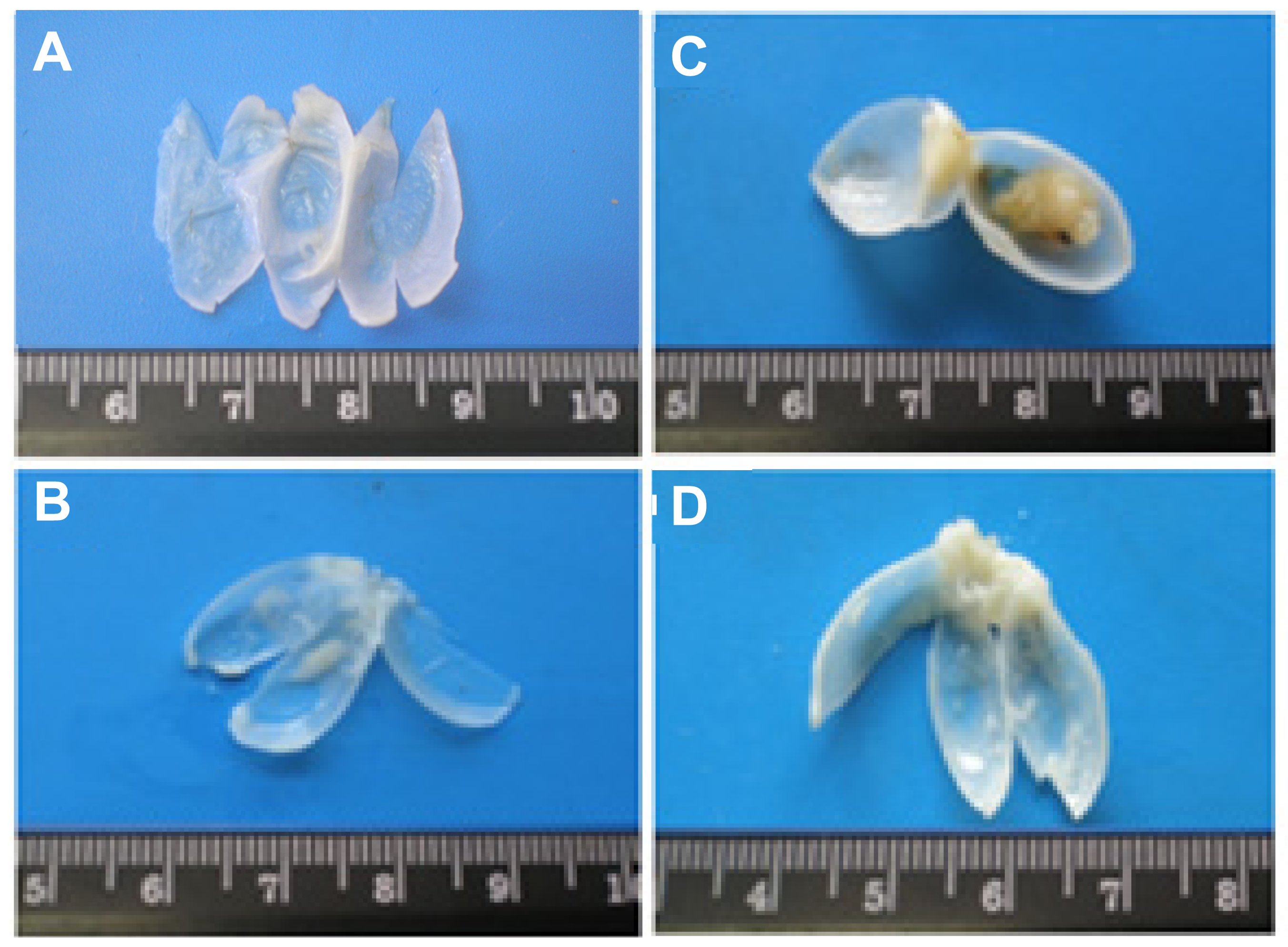
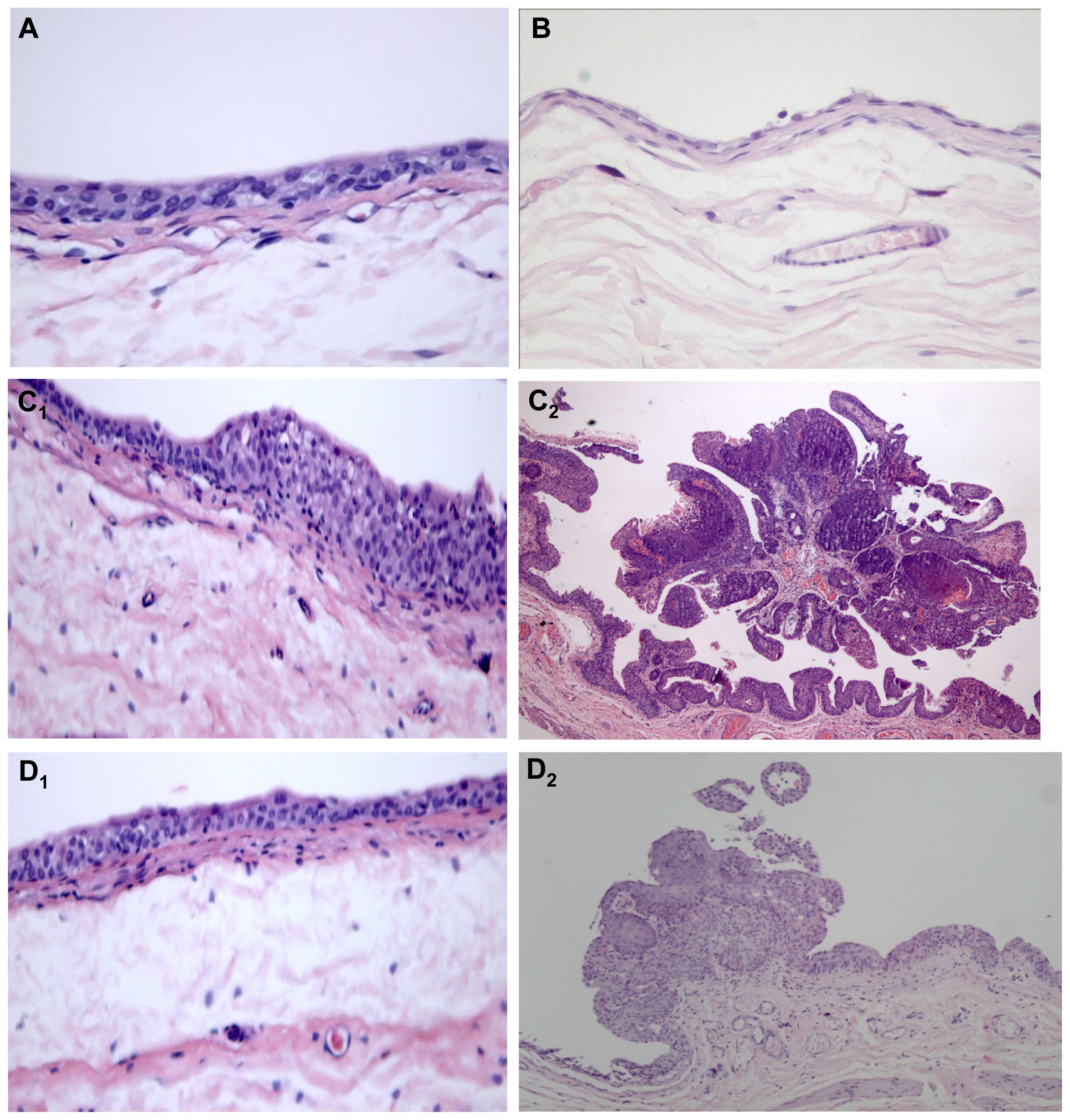

| Macroscopy (quantitative) | Control (n = 8) | Atorva (n = 8) | BBN (n = 20) | Atorva + BBN (n = 8) | ||
|---|---|---|---|---|---|---|
| Tumor number | ||||||
| % rats with tumor | 0 | 0 | 65.0% (13 in 20) | 12.5% (1 in 8) | ||
| Number tumors/rat | 0 | 0 | 1.2 ± 0.1 (16 in 13) | 2.0 (2 in 1) | ||
| Tumor volume (mm3): | ||||||
| Mean/rat | 0 | 0 | 138.5 ± 7.5 (in 13) | 4.7 (in 1) | ||
| Mean/tumor | 0 | 0 | 112.5 ± 6.4 (in 16) | 2.3 ± 0.2 (in 2) | ||
| Microscopy (qualitative) | Control | Atorva | BBN | Atorva + BBN | ||
| Tumors Group | Total Group | Tumors Group | Total Group | |||
| Pre-neoplastic lesions | ||||||
| Hyperplasia | 0 | 0 | 100 (13/13) | 100 (20/20) | 100 (1/1) | 38 (3/8) |
| High-grade dysplasia | 0 | 0 | 100 (13/13) | 75 (15/20) | 100 (1/1) | 13 (1/8) |
| Low-grade dysplasia | 0 | 0 | 0 (0/13) | 25 (5/20) | 0 (0/1) | 25 (2/8) |
| Neoplastic lesions | ||||||
| Papillary tumor | 0 | 0 | 100 (13/13) | 65 (13/20) | 100 (1/1) | 13 (2/8) |
| Infiltrative tumor | 0 | 0 | 15 (2/13) | 10 (2/20) | 0 (0/1) | 0 (0/8) |
| Carcinoma in situ | 0 | 0 | 31 (4/13) | 20 (4/20) | 0 (0/1) | 0 (0/8) |
| Parameters | Control (n = 8) | Atorva (n = 8) | BBN (n = 20) | Atorva + BBN (n = 8) |
|---|---|---|---|---|
| Renal function | ||||
| Creatinine (μmol/L) | 50.39 ± 0.88 | 56.57 ± 0.88 | 54.81 ± 1.77 | 59.23 ± 3.54 |
| Urea (mmol/L) | 6.40 ± 0.31 | 5.99 ± 0.32 | 6.27 ± 0.16 | 6.85 ± 0.24 |
| Liver function | ||||
| AST (IU/L) | 51.57 ± 1.09 | 54.00 ± 2.31 | 76.78 ± 4.40 aaa | 111.25 ± 5.72 bbb |
| ALT (IU/L) | 30.86 ± 1.75 | 31.00 ± 1.00 | 36.17 ± 2.32 | 53.43 ± 6.10 bb |
| CK activity (U/L) | 165.80 ± 14.60 | 697.67 ± 59.22 a | 231.88 ± 8.22 | 1132.17 ± 206.39 bbb |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Parada, B.; Reis, F.; Pinto, Â.; Sereno, J.; Xavier-Cunha, M.; Neto, P.; Rocha-Pereira, P.; Mota, A.; Figueiredo, A.; Teixeira, F. Chemopreventive Efficacy of Atorvastatin against Nitrosamine-Induced Rat Bladder Cancer: Antioxidant, Anti-Proliferative and Anti-Inflammatory Properties. Int. J. Mol. Sci. 2012, 13, 8482-8499. https://doi.org/10.3390/ijms13078482
Parada B, Reis F, Pinto Â, Sereno J, Xavier-Cunha M, Neto P, Rocha-Pereira P, Mota A, Figueiredo A, Teixeira F. Chemopreventive Efficacy of Atorvastatin against Nitrosamine-Induced Rat Bladder Cancer: Antioxidant, Anti-Proliferative and Anti-Inflammatory Properties. International Journal of Molecular Sciences. 2012; 13(7):8482-8499. https://doi.org/10.3390/ijms13078482
Chicago/Turabian StyleParada, Belmiro, Flávio Reis, Ângela Pinto, José Sereno, Maria Xavier-Cunha, Paula Neto, Petronila Rocha-Pereira, Alfredo Mota, Arnaldo Figueiredo, and Frederico Teixeira. 2012. "Chemopreventive Efficacy of Atorvastatin against Nitrosamine-Induced Rat Bladder Cancer: Antioxidant, Anti-Proliferative and Anti-Inflammatory Properties" International Journal of Molecular Sciences 13, no. 7: 8482-8499. https://doi.org/10.3390/ijms13078482




