Structural and Oxidative Changes in the Kidney of Crucian Carp Induced by Silicon-Based Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
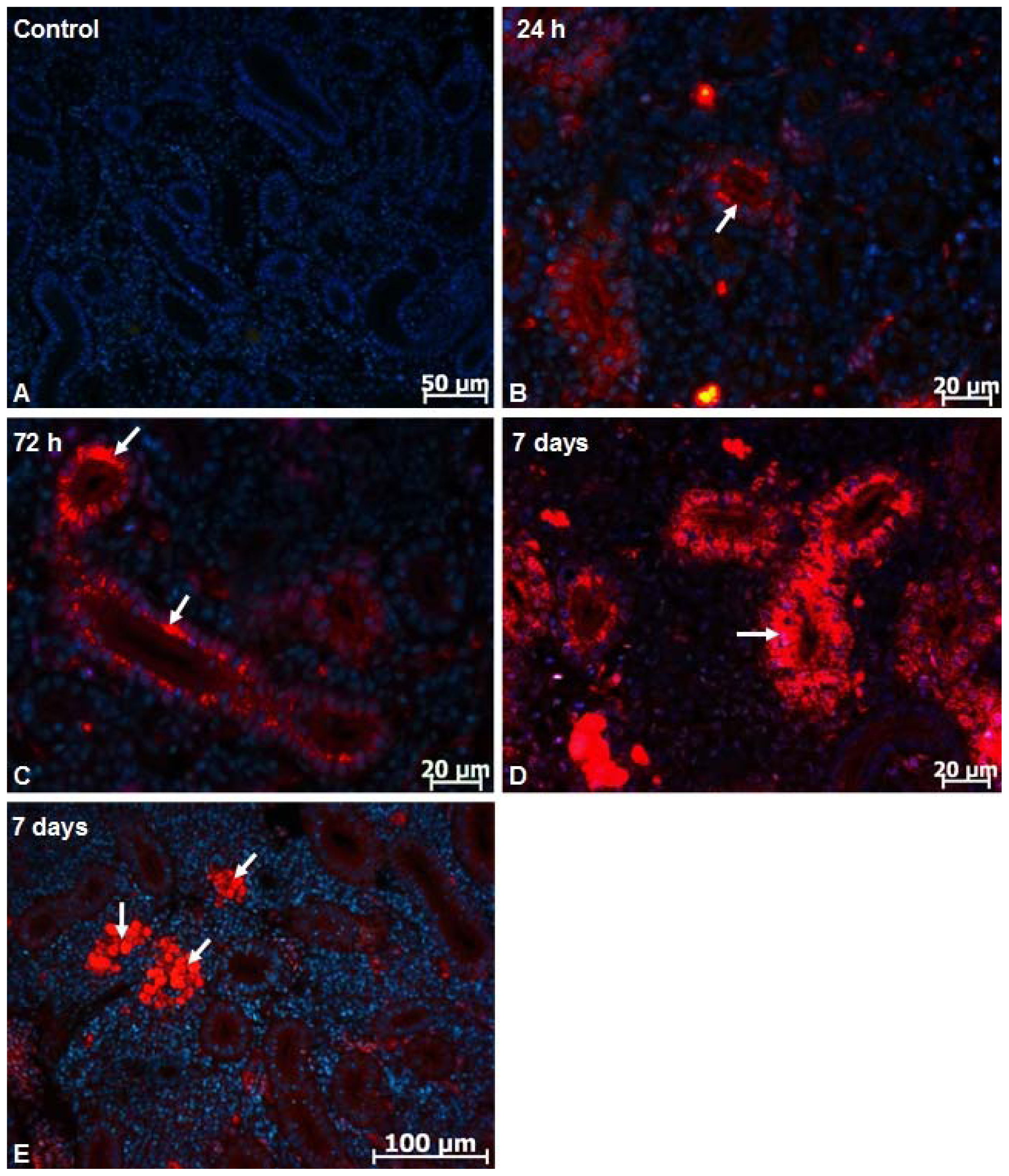
2.1. Microscopy Studies
2.2. Biochemical Studies
2.2.1. Lipid Peroxidation
2.2.2. GSH Level
2.2.3. Oxidative Protein Alterations
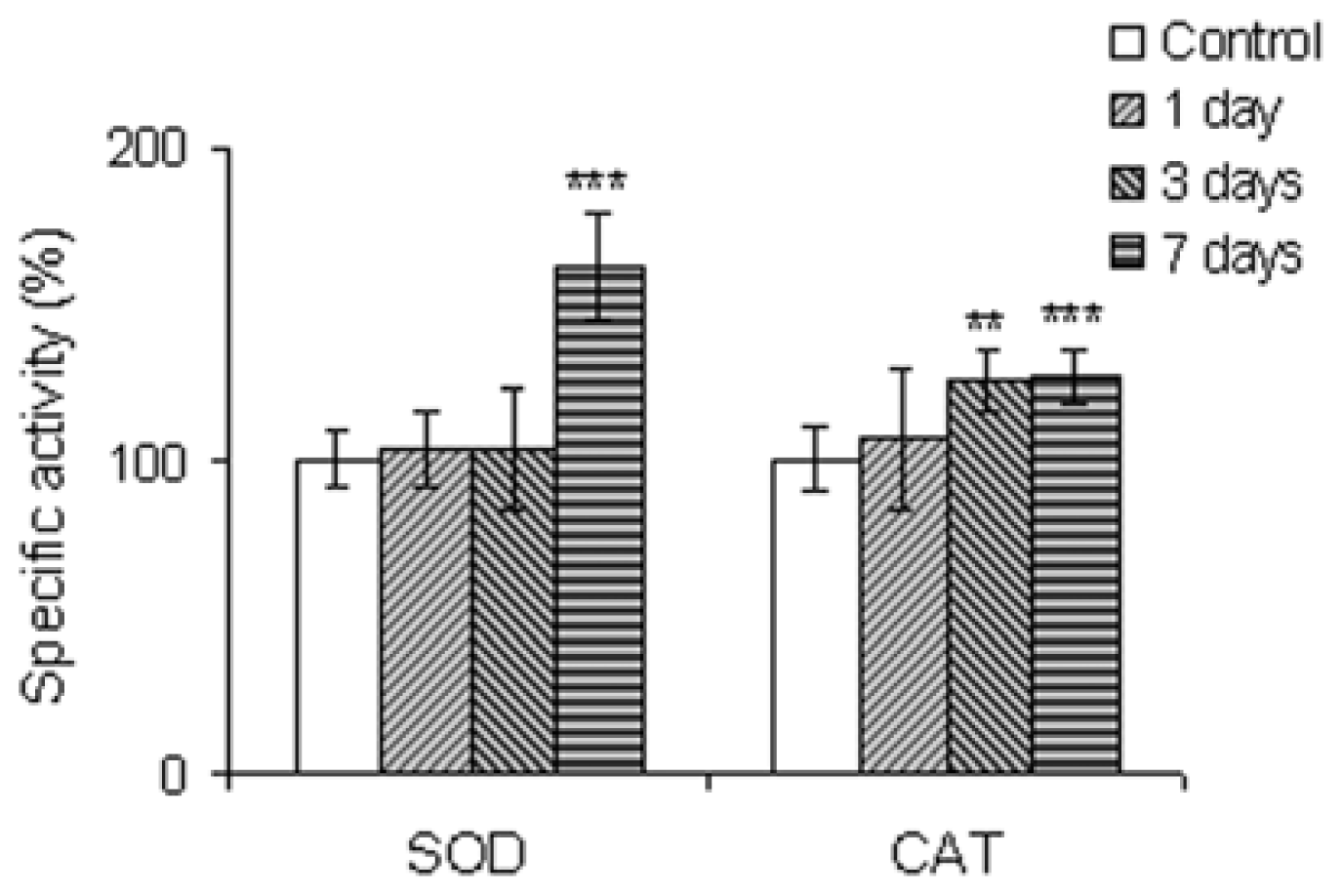
2.2.4. The Antioxidant Scavenging Enzymes
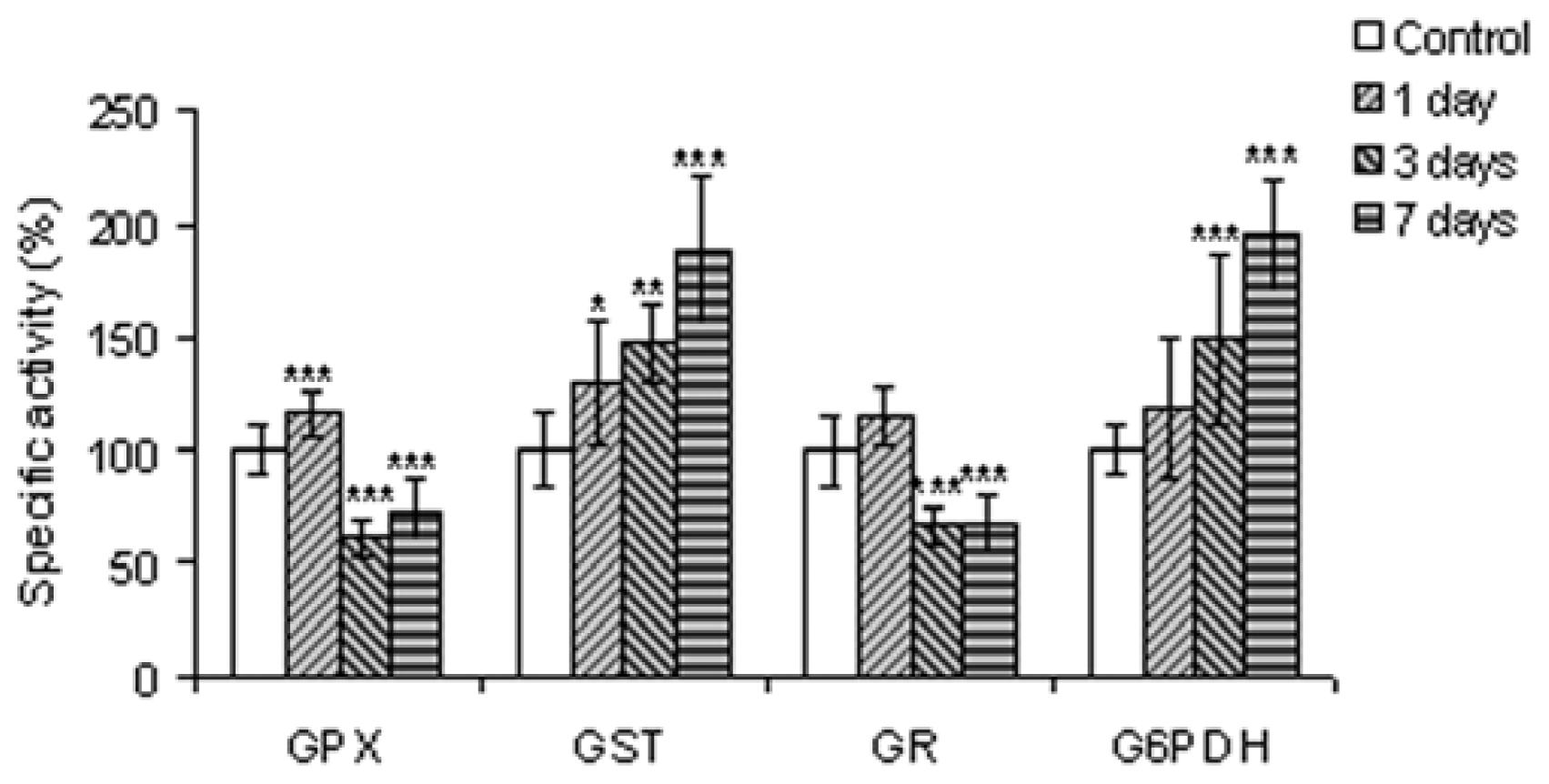
2.2.5. Enzymes Involved in Glutathione Metabolism and in Generating Reducing Equivalents
2.3. Heat Shock Protein Hsp 70 Evaluation
3. Experimental Section
3.1. Chemicals
3.2. Nanoparticles
3.3. Fish Maintenance and Treatments
3.4. Histology
3.5. Fluorescent Image Analysis of Nanoparticles Distribution
3.6. Preparation of Tissue Homogenates
3.7. Biochemical Analysis
3.7.1. Glutathione Assay
3.7.2. Malondialdehyde Assay
3.7.3. Protein Sulfhydryls Assay
3.7.4. Advanced Oxidation Protein Products
3.7.5. Protein Carbonyl Groups Assay
3.7.6. Enzyme Activity Assays
3.7.7. Protein Concentration
3.8. Western Blot Analysis of Hsp 70
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar]
- Vashist, S.K.; Tewari, R.; Bajpai, R.P.; Bharadwaj, L.M.; Raiteri, R. Review of quantum dot technologies for cancer detection and treatment. J. Nanotechnol. Online 2006, 2, 1–14. [Google Scholar]
- Chan, W.C.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminiscent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol 2002, 13, 40–46. [Google Scholar]
- Nida, D.L.; Nitin, N.; Yu, W.W.; Colvin, V.L.; Richards-Kortum, R. Photostability of quantum dots with amphiphilic polymer-based passivation. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Chen, C.-S.; Yao, J.; Durst, R.A. Liposome encapsulation of fluorescent nanoparticles: Quantum dots and silica nanoparticles. J. Nanop. Res 2007, 8, 1033–1038. [Google Scholar]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect 2006, 114, 165–172. [Google Scholar]
- Raisuddin, S.; Lee, J.-S. Fish Models in Impact Assessment of Carcinogenic Potential of Environmental Chemical Pollutants: An Appraisal of Hermaphroditic Mangrove Killifish Kryptolebias Marmoratus. In Interdisciplinary Studies on Environmental Chemistry-Biological Responses to Chemical Pollutants; Murakami, Y., Nakayama, K., Kitamura, S.-I., Iwata, H., Tanabe, S., Eds.; Terrapub: Tokyo, Japan, 2008; pp. 7–15. [Google Scholar]
- Paquet, D.; Bhat, R.; Sydow, A.; Mandelkow, E.M.; Berg, S.; Hellberg, S.; Fälting, J.; Distel, M.; Köstek, R.W.; Schmid, B.; et al. A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J. Clin. Invest 2009, 119, 1382–1395. [Google Scholar]
- Takashima, F.; Hibiya, T. An Atlas of Fish Histology: Normal and Pathological Features, 2nd ed; Kadansha: Tokyo, Japan, 1995. [Google Scholar]
- Thophon, S.; Kruatrachue, M.; Upathan, E.S.; Pokethitiyook, P.; Sahaphong, S.; Jarikhuan, S. Histopathological alterations of white seabass, Lates calcarifer in acute and subchronic cadmium exposure. Environ. Poluttion 2003, 121, 307–320. [Google Scholar]
- Augusto, J.; Smith, B.; Smith, S.; Robertson, J.; Reimschuessel, R. Gentamicin-induced nephrotoxicity and nephroneogenesis in Oreochromis nilotica, a tilapian fish. Dis. Aquat. Org 1996, 26, 49–58. [Google Scholar]
- Li, F.; Zhang, Z.-P.; Peng, J.; Ciu, Z.-Q.; Pang, D.-W.; Li, K.; Wei, H.-P.; Zhou, Y.-F.; Wen, J.-K.; Zhang, X.-E. Imaging viral behavior in mammalian cells with self- assembled capsid- quantum dot hybrid particles. Small 2009, 5, 718–726. [Google Scholar]
- Jain, R.K.; Stroh, M. Zooming in and out with quantum dots. Nat. Biotechnol 2004, 22, 959–960. [Google Scholar]
- Stanca, L.; Petrache, S.N.; Radu, M.; Serban, A.I.; Munteanu, M.C.; Teodorescu, D.; Staicu, A.C.; Sima, C.; Costache, M.; Grigoriu, C.; et al. Impact of silicon-based quantum dots on the antioxidative system in white muscle of Carassius auratus gibelio. Fish. Physiol. Biochem 2012, 38, 963–975. [Google Scholar]
- Erogbogbo, F.; Yon, K.-T.; Roy, I.; Xu, G.; Prasad, P.N.; Swihart, M.T. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2008, 2, 873–878. [Google Scholar]
- Kim, J.S.; Yoon, T.J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci 2006, 89, 338–347. [Google Scholar]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar]
- Liu, T.; Li, L.; Teng, X.; Huang, X.; Liu, H.; Chen, D.; Ren, J.; He, J.; Tang, F. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials 2011, 32, 1657–1668. [Google Scholar]
- Xie, G.; Sun, J.; Zhong, G.; Shi, L.; Zhang, D. Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch. Toxicol 2010, 84, 183–190. [Google Scholar]
- Scown, T.M.; Santos, E.M.; Johnston, B.D.; Gaiser, B.; Baalousha, M.; Mitov, S.; Lead, J.R.; Stone, V.; Fernandes, T.F.; Jepson, M.; et al. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol. Sci 2010, 115, 521–534. [Google Scholar]
- Scown, T.M.; van Aerle, R.; Johnston, B.D.; Cumberland, S.; Lead, J.R.; Owen, R.; Tyler, C.R. High doses of intravenously administered titanium dioxide nanoparticles accumulate in the kidneys of rainbow trout but with no observable impairment of renal function. Toxicol. Sci 2009, 109, 372–380. [Google Scholar]
- Cho, M.; Cho, W.S.; Choi, M.; Kim, S.J.; Han, B.S.; Kim, S.H.; Kim, H.O.; Sheen, Y.Y.; Jeong, J. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol. Lett 2009, 189, 177–183. [Google Scholar]
- Choi, C.H.J.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar]
- Reimschuessel, R. A fish model of renal regeneration and development. ILAR J 2001, 42, 285–291. [Google Scholar]
- Hinton, D.E.; Laurén, D.J. Integrative histopathological approaches to detecting effects of environmental stressors on fishes. Am. Fish. Soc. Symp 1990, 8, 51–66. [Google Scholar]
- Reimschuessel, R.; Bennett, R.O.; May, E.B.; Lipsky, M.M. Development of newly formed nephrons in the goldfish kidney following hexachlorobutadiene-induced nephrotoxicity. Toxicol. Pathol 1990, 18, 32–38. [Google Scholar]
- Reimschuessel, R.; Bennett, R.O.; May, E.A.; Lipsky, M.M. Pathological alterations and new nephron development in rainbow trout Oncorhyncus mykiss following tetrachloroethylene contamination. J. Zoo Anim. Med 1993, 24, 503–507. [Google Scholar]
- Watanabe, N.; Kato, M.; Suzuki, N.; Inoue, C.; Fedorova, S.; Hashimoto, H.; Maruyama, S.; Matsuo, S.; Wakamatsu, Y. Kidney regeneration through nephron neogenesis in medaka. Dev. Growth Differ 2009, 51, 135–143. [Google Scholar]
- Iles, K.E.; Forman, H.J. Macrophage signaling and respiratory burst. Immunol. Res 2002, 26, 95–105. [Google Scholar]
- Gill, P.S.; Wilcox, C.S. NADPH oxidases in the kidney. Antioxid. Redox Signal 2006, 8, 1597–1607. [Google Scholar]
- Geiszt, M.; Kopp, J.B.; Varnai, P.; Leto, T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med 1999, 29, 916–921. [Google Scholar]
- Schnellmann, R.G.; Gilchrist, S.M.; Mandel, L. Intracellular distribution and depletion of glutathione in rabbit renal proximal tubules. Kidney Int 1988, 34, 229–233. [Google Scholar]
- Liu, R.-M.; Gao, L.; Choi, J.; Forman, H.J. γ-Glutamylcysteinil synthetase mRNA stabilization and independent subunit transcription by 4-hydroxy-2-nonenal. Am. J. Physiol 1998, 275, 861–869. [Google Scholar]
- Gianazza, E.; Crawford, J.; Miller, I. Detecting oxidative post-translational modifications in proteins. Amino Acids 2007, 33, 51–60. [Google Scholar]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Ngnyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Yungers, P.; Deschamps-Latecha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996, 49, 1304–1313. [Google Scholar]
- Alderman, C.J.; Shah, S.; Foreman, J.C.; Cham, B.M.; Katz, D.R. The role of advanced oxidation protein products in regulation of dendritic cell function. Free Radic. Biol. Med 2002, 32, 377–385. [Google Scholar]
- Schachter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev 2000, 32, 307–326. [Google Scholar]
- Aksenov, M.Y.; Aksenova, M.V.; Butterfield, D.A.; Geddes, J.W.; Markesbery, W.R. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience 2001, 103, 373–383. [Google Scholar]
- Miyata, T.; van Ypersele de Stribou, C.; Kurokawa, K.; Baynes, J.W. Alterations in nonenzymatic biochemistry in uremia: Origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int 1999, 55, 389–399. [Google Scholar]
- Schröder, E.; Eaton, P. Hydrogen Peroxide and Cysteine Protein Signaling Pathways. In Redox Signaling and Regulation in Biology and Medicine; Jacob, C., Winyard, P.G., Eds.; Wiley-VCH Verlag GmbH &Co: Weinheim, Germany, 2009; p. 189. [Google Scholar]
- Dalle Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction and disease progression. J. Cell Mol. Med 2006, 10, 389–406. [Google Scholar]
- Oruc, E.Ö.; Sevgiler, Y.; Uner, N. Tissue-Specific oxidative stress responses in fish exposed to 2,4-D and azinphosmethyl. Comp. Biochem. Physiol. 2004, 137, 43–51. [Google Scholar]
- Asagba, S.O.; Eriyamremu, G.E.; Igberaese, M.E. Bioaccumulation of cadmium and its biochemical effect on selected tissues of the catfish (Clariasgariepinus). Fish Physiol. Biochem 2008, 34, 61–69. [Google Scholar]
- Rozell, B.; Hansson, H.A.; Guthenberg, C.; Kalim Tahir, M.; Mannervik, B. Glutathione transferases of classes alpha, mu and pi show selective expression in different regions of rat kidney. Xenobiotica 1993, 23, 835–849. [Google Scholar]
- Beutler, E.; Vulliamy, T.; Luzzatto, L. Hematologically important mutations: Glucose-6-phosphate dehydrogenase. Blood Cells Mol. Dis 1996, 22, 49–76. [Google Scholar]
- Renfro, J.L.; Brown, M.A.; Parker, S.L.; Hightower, L.E. Relationship of thermal and chemical tolerance to transepithelial transport by cultured flounder renal epithelium. J. Pharmacol. Exp. Therap 1993, 265, 992–1000. [Google Scholar]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci 2010, 86, 377–384. [Google Scholar]
- Kiang, J.G.; Tsokos, G.C. Hsp 70 kDa: Molecular biology, biochemistry and physiology. Pharmacol. Therapeut 1998, 80, 183–201. [Google Scholar]
- Parcellier, A.; Gurbuxani, S.; Schmitt, E.; Solary, E.; Garrido, C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem. Biophys. Res. Commun 2003, 304, 505–512. [Google Scholar]
- Cumming, R.C.; Andon, N.L.; Haynes, P.A.; Minkyu, P.; Fischer, W.H.; Schubert, D. Protein disulphide bond formation in the cytoplasm during oxidative stress. J. Biol. Chem 2004, 279, 21749–21758. [Google Scholar]
- Kayhan, F.E.; Duman, B.S. Heat shock protein genes in fish. Turk. J. Fish. Aquat. Sci 2010, 10, 287–293. [Google Scholar]
- Duffy, L.K.; Scofield, E.; Rodgers, T.; Patton, M.; Bowyer, R.T. Comparative baseline levels of mercury, Hsp70 and Hsp 60 in subsistence fish from the Yukon-Kuskokwim delta region of Alaska. Comp. Biochem. Physiol 1999, 124, 181–186. [Google Scholar]
- Scheil, V.; Zürn, A.; Kohler, H.R.; Riebskorn, R. Embryo development, stress protein (Hsp 70) responses, and histopathology in zebrafish (Danio rerio) following exposure to nickel chloride, chlorpyrofos, and binary mixtures of them. Environ. Toxicol 2010, 25, 83–93. [Google Scholar]
- Chen, J.; Dong, X.; Zhao, J.; Tang, G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitoneal injection. J. Appl. Toxicol 2009, 29, 330–337. [Google Scholar]
- Grigoriu, C.; Nicolae, I.; Ciupina, V.; Prodan, G.; Suematsu, H.; Yatsui, K. Influence of the experimental parameters on silicon nanoparticles produced by laser ablation. J. Optoelectr. Adv. Mat 2004, 6, 825–830. [Google Scholar]
- Grigoriu, C.; Kuroki, Y.; Nicolae, I.; Zhu, X.; Hirai, M.; Suematsu, H.; Takata, M.; Yatsui, K. Photo and cathodoluminescence of Si/SiO2 nanoparticles produced by laser ablation. J. Optoelectr. Adv. Mat 2005, 7, 2979–2984. [Google Scholar]
- Yatsui, K.; Yukawa, T.; Grigoriu, C.; Hirai, M.; Jiang, W. Synthesis of ultrafine gamma-Al2O3 powders by pulsed laser ablation. J. Nanop. Res 2000, 2, 75–83. [Google Scholar]
- Grigoriu, C.; Hirai, M.; Nishiura, K.; Jiang, W.; Yatsui, K. Synthesis of nanosized aluminium nitride powders by pulsed laser ablation. J. Am. Ceram. Soc 2000, 83, 2631–2633. [Google Scholar]
- Del Rio, D.; Pellegrini, N.; Colombi, B.; Bianchi, M.; Serafini, M.; Torta, F.; Tegoni, M.; Musci, M.; Brighenti, F. Rapid fluorimetric method to detect total plasma malondialdehyde with mild derivatization conditions. Clin. Chem 2003, 49, 690–692. [Google Scholar]
- Riener, C.; Kada, G.; Gruber, H.J. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4′-dithiodipyridine. Anal. Bioanal. Chem 2002, 373, 266–276. [Google Scholar]
- Witko-Sarsat, V.; Nguyen, A.T.; Descamp, S.; Latsha, B. Microtitre plate assay for phagocyte derived taurine chloroaminea. J. Clin. Lab. Ann 1992, 6, 47–53. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A. Determination of carbonyl content in oxidaively modified protein. Methods Enzymol 1990, 186, 494–498. [Google Scholar]
- Paoletti, F.; Mocali, A. Determination of superoxide dismutase activity by purely chemical system based on NADP(H) oxidation. Methods Enzymol 1990, 186, 209–221. [Google Scholar]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–677. [Google Scholar]
- Lohr, G.W.; Waller, H.D. Glucose-6-Phosphate Dehydrogenase. In Methods of Enzymatic Analysis; Bergmeyer, H.V., Ed.; Academic Press: New York, NY, USA, 1974; pp. 744–751. [Google Scholar]
- Beutler, E. Red Cell Metabolism. In A Manual of Biochemical Methods; Beutler, E., Ed.; Grune and Stratton: Orlando, FL, USA, 1984; pp. 68–73. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem 1974, 249, 7130–7139. [Google Scholar]
- Goldberg, D.M.; Spooner, R.J. Glutathione Reductase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; Volume 111, pp. 258–265. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin-phenol reagents. J. Biol. Chem 1951, 193, 265–275. [Google Scholar]


| Time Interval | MDA (nmoles/mg) | GSH (nmoles/mg) | ||
|---|---|---|---|---|
| Control Group | Exposed Group | Control Group | Exposed Group | |
| 1 day | 100 ± 7 | 197 ± 41 *** | 100 ± 6 | 34 ± 6 *** |
| 3 days | 100 ± 11 | 388 ± 108 *** | 100 ± 10 | 40 ± 10 *** |
| 7 days | 100 ± 8 | 274 ± 82 *** | 100 ± 6 | 53 ± 8 ** |
| Protein Thiols (nmoles/mg) | AOPP (μmoles/mg) | PRCG (nmoles/mg) | ||||
|---|---|---|---|---|---|---|
| Time Interval | Control Group | Exposed Group | Control Group | Exposed Group | Control Group | Exposed Group |
| 1 day | 100 ± 19 | 72 ± 12 * | 100 ± 8 | 111 ± 12 | 100 ± 22 | 95 ± 18 |
| 3 days | 100 ± 20 | 67 ± 15 * | 100 ± 13 | 134 ± 26 * | 100 ± 7 | 222 ± 55 *** |
| 7 days | 100 ± 18 | 57 ± 7 ** | 100 ± 10 | 110 ± 16 | 100 ± 27 | 215 ± 56 *** |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Petrache, S.N.; Stanca, L.; Serban, A.I.; Sima, C.; Staicu, A.C.; Munteanu, M.C.; Costache, M.; Burlacu, R.; Zarnescu, O.; Dinischiotu, A. Structural and Oxidative Changes in the Kidney of Crucian Carp Induced by Silicon-Based Quantum Dots. Int. J. Mol. Sci. 2012, 13, 10193-10211. https://doi.org/10.3390/ijms130810193
Petrache SN, Stanca L, Serban AI, Sima C, Staicu AC, Munteanu MC, Costache M, Burlacu R, Zarnescu O, Dinischiotu A. Structural and Oxidative Changes in the Kidney of Crucian Carp Induced by Silicon-Based Quantum Dots. International Journal of Molecular Sciences. 2012; 13(8):10193-10211. https://doi.org/10.3390/ijms130810193
Chicago/Turabian StylePetrache, Sorina Nicoleta, Loredana Stanca, Andreea Iren Serban, Cornelia Sima, Andreia Cristina Staicu, Maria Cristina Munteanu, Marieta Costache, Radu Burlacu, Otilia Zarnescu, and Anca Dinischiotu. 2012. "Structural and Oxidative Changes in the Kidney of Crucian Carp Induced by Silicon-Based Quantum Dots" International Journal of Molecular Sciences 13, no. 8: 10193-10211. https://doi.org/10.3390/ijms130810193




