2.1. Effects of Humidity on the Melt Crystallization of IBU
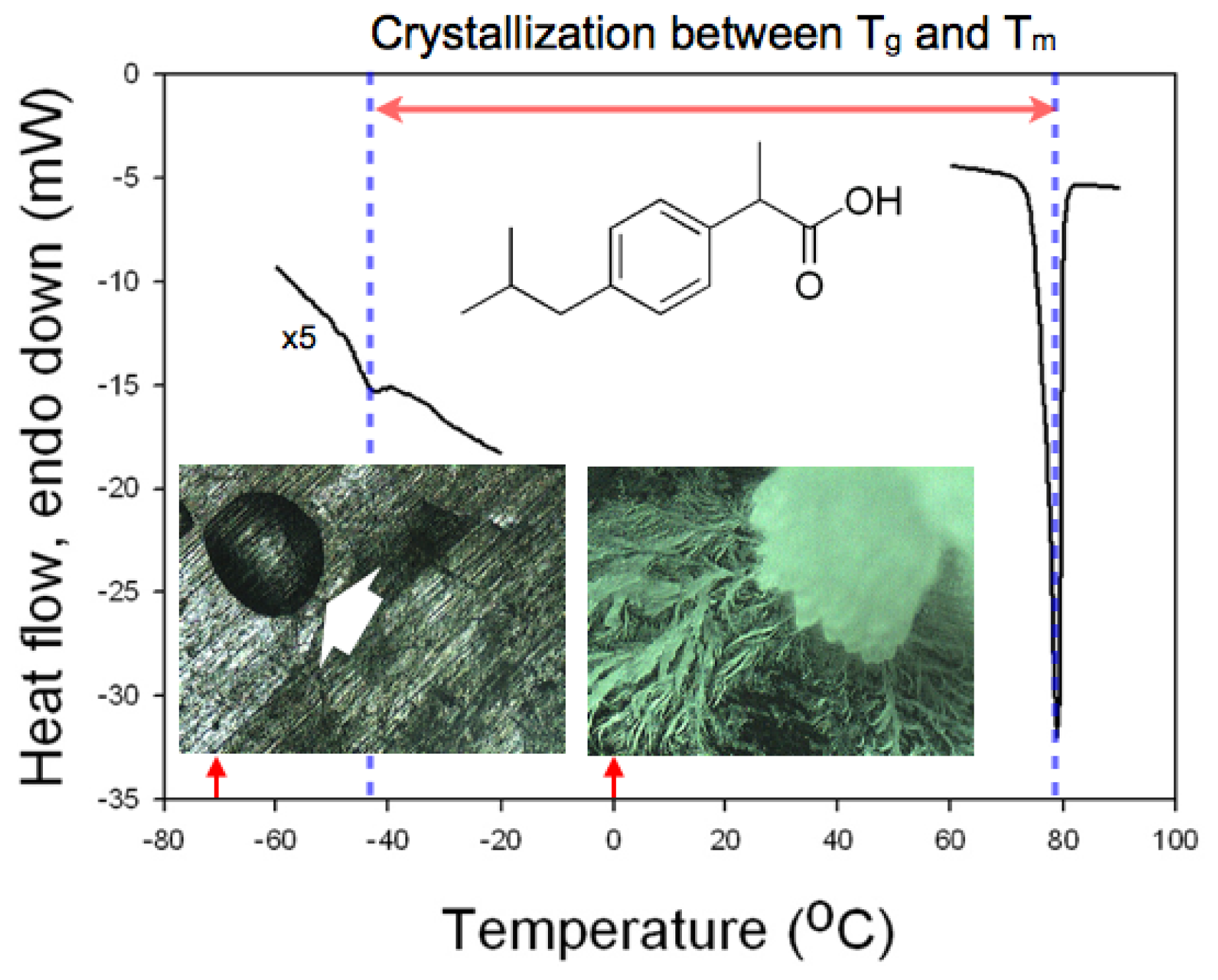
Differential scanning calorimetry (DSC) was performed to confirm the temperature range that could be utilized for the crystallization of IBU. The two limiting points that defined the temperature range were taken as the glass transition temperature and the melting point of IBU [
12]. The former decided the temperature below which the mobility of the IBU molecules would be prohibitively low to initiate the molecular self-assembly that would be necessary to form IBU nuclei. The latter defined the temperature where the supercooling, the driving force for the melt crystallization, would be nil. The glass transition temperature and the melting point of IBU were found as −42.3 °C and 78.9 °C, respectively, with the scanning rate of 10 °C/min (
Figure 1), which was in accordance with the previous studies [
13,
14]. For example, as shown in the micrographs in
Figure 1, when IBU melt was placed at −70 °C, no crystallization occurred even after 12 h (amorphous IBU denoted by a white arrow), while crystallization of IBU melt was complete at 0 °C under the same conditions otherwise. Based on the DSC study, we chose room temperature (18–22 °C) for the crystallization experiments with controlled relative humidity (RH) because it was about the center of the glass transition temperature and the melting point, where sufficient mobility and supercooling were provided.
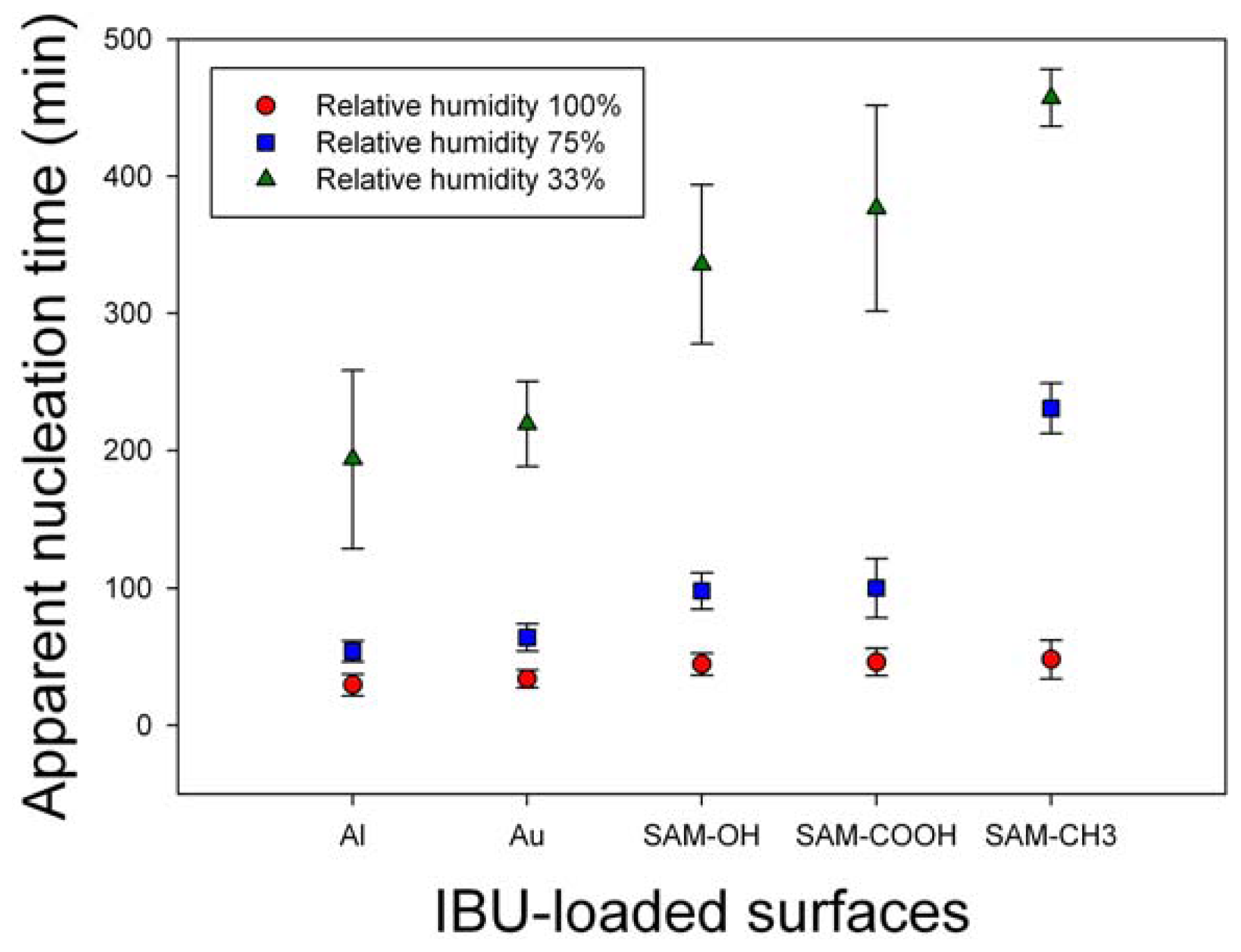
The effects of humidity on IBU melt crystallization were monitored in terms of nucleation, crystallinity, crystal phase, and morphology. First, the effect on the nucleation was summarized in
Figure 2. Nucleation time was monitored at RH 33%, 75%, and 100%, and the crystallization was on Al, Au, and self-assembled monolayers with –OH, –COOH, and –CH
3 functional groups (SAM–OH, SAM–COOH, and SAM–CH
3, respectively). Each result was the average of eight independent experiments. On all surfaces, the observation time of the initial nucleation was inversely proportional to RH,
i.e., the nucleation time was in the order, RH 100% < 75% < 33%. Also, the nucleation time was similar for all surfaces at RH 100%, and it was relatively shorter on metal surfaces and longer on SAM surfaces (especially SAM–CH
3) at RH 75% and 33%. Overall, we could conclude that humidity, probably through the adsorbed water molecules at the melt/atmosphere interface, acted on the IBU melts to initiate the molecular assembly and start the nucleation process. Although the exact mechanism through which the water molecules affected the metastable IBU melts is not clear at this point, it could be through the increased instability. The adsorbed water molecules on the metastable IBU melts would replace some of the existing IBU melt/air interface with the melt/water interface. The new interface is most likely less favorable since water is immiscible with IBU melt, and it is a very poor solvent of IBU (solubility 0.011 mg/mL at 25 °C and pH 7.4) [
15,
16]. Therefore, the newly formed and unfavorable interface would prompt the metastable melts to nucleate a denser crystal phase to reduce the interfacial area of IBU/water. The nucleating effect of water was also confirmed by observing the immediate crystallization when the IBU melt was in contact with liquid water (data now shown).
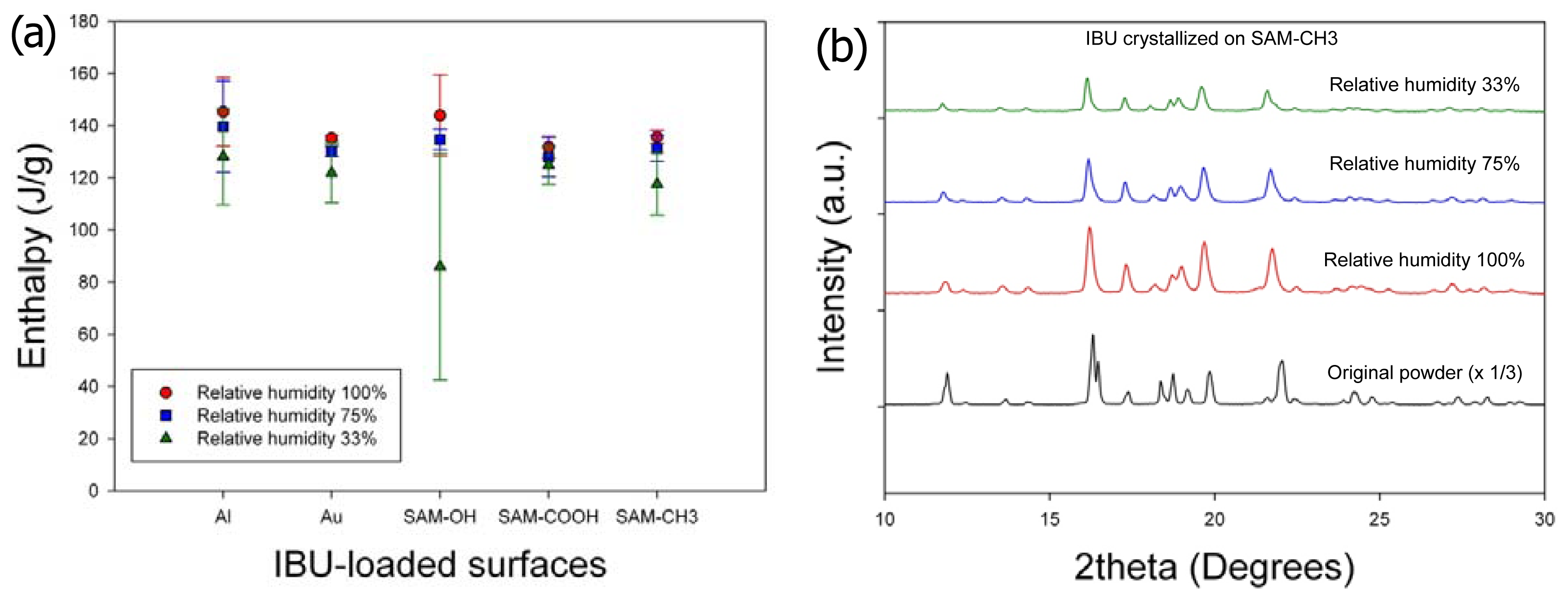
Second, the effects of humidity on the crystallinity and crystal phase (or polymorph) of IBU were minimal (
Figure 3). The melting enthalpy of the samples, 1 day after crystallization, was summarized in
Figure 3a (three independent experiments). Slight increase in the heat of fusion along with humidity increase was within the range of standard deviations, and any correlation with the surfaces was hard to establish. (Note that the melting enthalpy of the original powder was 141.0 ± 5.9 J/g.) The melting points of all the obtained crystals were located in the range
ca. 75 °C–78 °C, suggesting that the obtained crystals were of the same crystal polymorph. (Note that the melting point of the original powder was 78.9 °C.) This was also confirmed by powder X-ray diffraction (PXRD) patterns, as the characteristic diffraction peaks were identical among the purchased powders and all the recrystallized samples on all surfaces. Representative results were shown in
Figure 3b for the IBU recrystallized on SAM–CH
3 along with the originally purchased IBU powders, and the results were the same for the IBU recrystallized under all the other conditions. Finally, the effect of humidity on the IBU crystal morphology was negligible. Again, the representative case was shown for the IBU recrystallized on SAM–CH
3 (
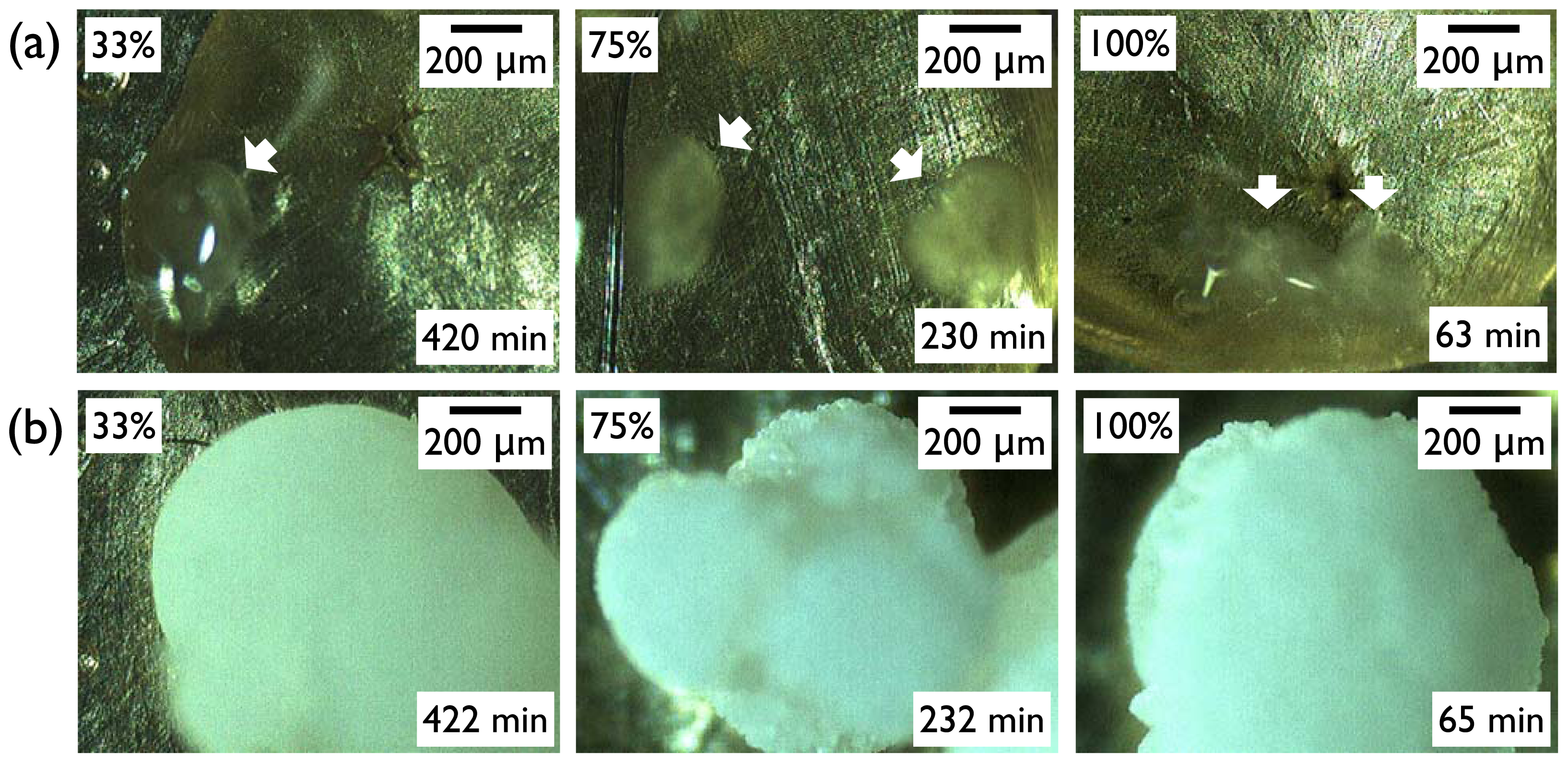
Figure 4). Top pictures (
Figure 4a) were taken as soon as the turbid regions appeared within the clear area of IBU melts. Note that the
in situ OM observation was made through a clear polystyrene chamber (diameter 9 cm, height 1.5 cm, SPL Lifesciences) connected through a rubber vacuum tube to a glass desiccator (diameter 14 cm, height 16 cm) of the controlled RH. Within a few minutes of initial crystal formation, the crystal growth appeared to complete as shown in the bottom pictures (
Figure 4b). The morphology was more or less spherical for the IBU recrystallized on SAM–CH
3 irrespective of the relative humidity. (Note that the morphologies of the IBU crystals were not the same among the various crystallization surfaces, and the results were summarized in the next section. In the current section, the effect of the various surfaces was intentionally omitted to focus on the influence of humidity.) From these results, we could conclude that the effects of humidity was largely limited to the nucleation of IBU crystals, and the crystallinity, crystal phase, and morphology of IBU were on the whole invariant with the change of relative humidity.
2.2. Effects of Surfaces on the Melt Crystallization of IBU
To study the effects of the various surfaces, the melt crystallization of IBU was performed at −20 °C, rather than room temperature as was in the pervious section. This condition was chosen to make it easy to monitor the growth behavior on the various surfaces because it ensured a relatively slower crystal growth after nucleation, probably due to the combined effects of the low temperature and reduced humidity. The very low atmospheric water content also made it possible to examine the effects of surfaces with a minimal influence of humidity. (The water content is only 0.23 g/kg dry air at this condition (−20 °C, RH 36%) compared to 4.75 g/kg dry air at 20 °C and RH 33% [
17]). Also, the IBU crystal morphology was found to be virtually the same for the crystallization at −20 °C, 0 °C, and room temperature for the same surface.
In this experiment, multiple IBU melts on the same type of surfaces were placed in the −20 °C freezer in the beginning, and each sample was taken out at a given crystallization time to microscopically observe the crystalline area out of the entire melt area. Then, the crystallization behavior was analyzed through the Avrami equation:
where
Xc(
t) is the relative crystallinity at time
t,
n is the Avrami exponent, and
K is the rate constant. Since the Avrami equation is based on the spatial profile of molecular assembly on the crystal surfaces, the Avrami-type analysis of the crystallization kinetics provides important information on the assembly and the resulting crystal morphology [
12,
18].
While the microscopic measurement of the crystallinity in the middle of crystal growth was difficult to assess accurately because of the three-dimensional nature of the crystals, projected area of the crystals out of the entire melt region was roughly taken as the relative crystallinity,
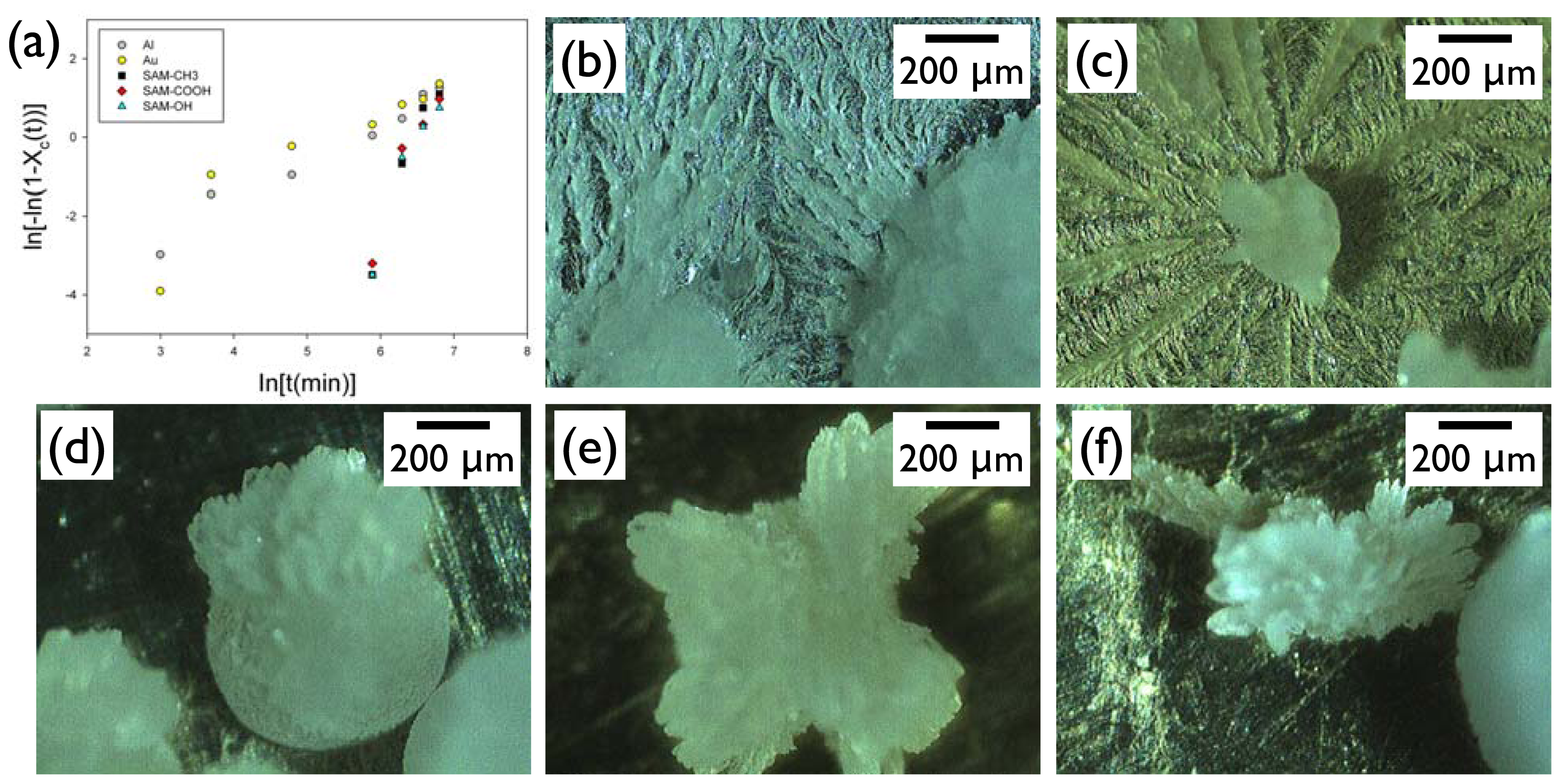
Xc. This analysis clearly revealed that the Al and Au surfaces exhibited completely different behavior from the SAM surfaces (
Figure 5a). On these high-energy metal surfaces, the crystallization started more quickly (<20 min) than on the SAM surfaces, and it appeared to have two sequential stages: (i) initially rapid growth; (ii) later slower propagation stages. The Avrami exponent for the IBU growth on Al and Au of the initial stage was
ca. 2.2–4.3, and that of the later stage was 0.76–1.4. In contrast, the crystallization started only after about 360 min on the SAM surfaces, and it was complete relatively quickly. The Avrami exponents for the IBU growth on the SAM-OH, SAM-COOH, and SAM-CH
3 were
ca. 4.5–5.2. Note that the theoretical Avrami exponents for the spherical growth are 3 or 4 depending on the preexistence or constant formation of the nuclei, for the fibrillar growth less than 1 or 2, and for sheaf-like growth more than 5 [
12]. The analysis of the growth kinetics based on the Avrami equation was in good agreement with the morphology observation. The IBU on Al or Au (
Figure 5b,c) consisted of the approximately spherical crystals surrounded by the fibrillar structures closely attached to the metal surfaces. The IBU on SAM surfaces (
Figure 5d–f) only exhibited bulk crystals, of which end structures were often bundled needles or sheaf-like, and no fibrillar formation was found on the SAM surfaces. From these results, we could conclude that the surfaces affected both nucleation and growth of IBU crystals. The nucleation time was relatively faster on the metal surfaces, which was in accordance with the results of Section 2.1 (e.g., see the low-humidity case). The growth kinetics was profoundly different between metals and SAMs. The former surfaces induced initially spherical and later fibrillar growth. The latter formed a combined morphology of spherical and sheaf-like crystals. This difference seemed to arise from the balanced interactions of the IBU melt with the surfaces and the growth front of the crystals [
19]. Experiments and calculations are currently underway to explore the nature of these interactions.