Neuroprotective Effects of Triterpene Glycosides from Glycine max against Glutamate Induced Toxicity in Primary Cultured Rat Cortical Cells
Abstract
:1. Introduction
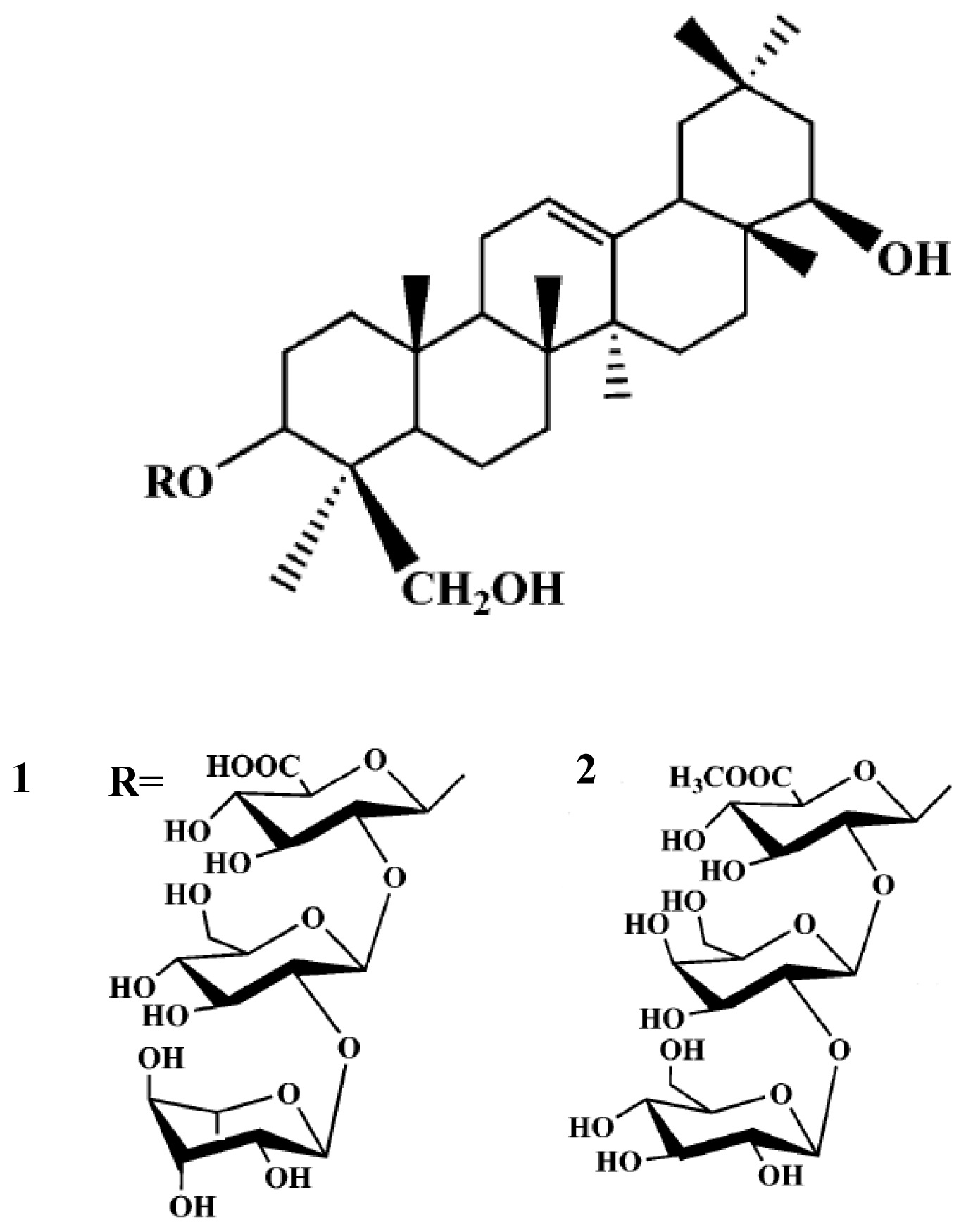
2. Results and Discussion
3. Experimental Section
3.1. Plant Material
3.2. Extraction and Isolation of Compounds
3.3. Neuroprotective Activity Testing
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Aparicio-Fernández, X.; García-Gasca, T.; Yousef, G.G.; Lila, M.A.; González de Mejia, E.; Loarca-Pina, G. Chemopreventive activity of polyphenolics from black Jamapa bean (Phaseolus vulgaris L.) on HeLa and HaCaT cells. J. Agric. Food Chem 2006, 54, 2116–2122. [Google Scholar]
- Iriti, M.; di Maro, A.; Bernasconi, S.; Burlini, N.; Simonetti, P.; Picchi, V.; Panigada, C.; Gerosa, G.; Parente, A.; Faoro, F. Nutritional traits of bean (Phaseolus vulgaris) seeds from plants chronically exposed to ozone pollution. J. Agric. Food Chem 2009, 57, 201–208. [Google Scholar]
- Hangen, L.; Bennink, M.R. Consumption of black beans and navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer 2002, 44, 60–65. [Google Scholar]
- Duodu, K.G.; Nunes, A.; Delgadillo, I.; Parker, M.L.; Mills, E.N.C.; Belton, P.S. Effect of grain structure and cooking on sorghum and maize in vitro protein digestibility. J. Cereal Sci 2002, 35, 161–174. [Google Scholar]
- Wang, R.; Zhou, J.; Tang, X.C. Tacrine attenuates hydrogen peroxideinduced apoptosis by regulating expression of apoptosisrelated genes in rat PC12 cells. Mol. Brain Res 2002, 107, 1–8. [Google Scholar]
- Sucher, N.J.; Awobuluyi, M.; Choi, Y.B.; Lipton, S.A. NMDA receptors: From genes to channels. Trends Pharmacol. Sci 1996, 17, 348–355. [Google Scholar]
- Mizuno, T. The biphasic role of microglia in Alzheimer’s disease. Int. J. Alzheimer’s Dis 2012. [Google Scholar] [CrossRef]
- Koo, K.A.; Kim, S.H.; Oh, T.H.; Kim, Y.C. Acteoside and its aglycones protect primary cultures of rat cortical cells from glutamate-induced excitotoxicity. Life Sci 2006, 79, 709–716. [Google Scholar]
- Trist, D.G. Excitatory aminoacid agonists and antagonists: Pharmacology and therapeutic applications. Pharm. Acta Helvetiae 2000, 74, 221–229. [Google Scholar]
- Dong, M.; He, X.; Liu, R.H. Phytochemicals of black bean seed coats: Isolation, structure elucidation, and their antiproliferative and antioxidative activities. J. Agric. Food Chem 2007, 55, 6044–6051. [Google Scholar]
- Koo, K.A.; Lee, M.K.; Kim, S.H.; Jeong, E.J.; Kim, S.Y.; Oh, T.H.; Kim, Y.C. Pinusolide and 15-methoxypinusolidic acid attenuate the neurotoxic effect of staurosporine in primary cultures of rat cortical cells. Br. J. Pharmacol 2007, 150, 65–71. [Google Scholar]
| Dose | Cell Viability b,d (%) | ||
|---|---|---|---|
| 5 μg/mL | 25 μg/mL | 100 μg/mL | |
| Control c | 100 | ||
| Glutamate-treated c,e | 0 | ||
| MeOH extracts | 13.4 ± 0.3 | 38.5 ± 3.2 * | 59.0 ± 3.6 ** |
| Chloroform fraction | - | - | 12.3 ± 1.3 |
| Ethyl acetate fraction | - | 21.4 ± 6.3 * | 55.3 ± 5.7 ** |
| n-butanol fraction | - | - | 5.8 ± 1.3 |
| BB-1 subfraction | - | - | - |
| BB-2 subfraction | - | - | - |
| BB-3 subfraction | 15.6 ± 3.6 * | 36.7 ± 3.7 * | 43.5 ± 2.6 ** |
| BB-4 subfraction | - | - | 26.7 ± 8.9 |
| BB-5 subfraction | - | - | - |
| Dose | Cell Viability b,d (%) | ||
|---|---|---|---|
| 0.1 μM | 1 μM | 10 μM | |
| Control c | 100 | ||
| Glutamate-treated c,e | 0 | ||
| 1 | 14.2 ± 0.3 | 16.7 ± 1.2 | 21.4 ± 5.6 |
| 2 | 16.7 ± 1.5 * | 39.2 ± 1.5 ** | 71.5 ± 6.8 *** |
| APV f | 11.5 ± 1.4 | 26.5 ± 2.3 * | 42.5 ± 3.7 * |
| MK-801 g | 51.4 ± 4.6 ** | 63.5 ± 5.8 *** | 78.4 ± 2.0 *** |
| CNQX h | 23.5 ± 3.7 * | 44.8 ± 3.5 * | 53.2 ± 4.6 *** |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moon, H.-I.; Lee, J.-H. Neuroprotective Effects of Triterpene Glycosides from Glycine max against Glutamate Induced Toxicity in Primary Cultured Rat Cortical Cells. Int. J. Mol. Sci. 2012, 13, 9642-9648. https://doi.org/10.3390/ijms13089642
Moon H-I, Lee J-H. Neuroprotective Effects of Triterpene Glycosides from Glycine max against Glutamate Induced Toxicity in Primary Cultured Rat Cortical Cells. International Journal of Molecular Sciences. 2012; 13(8):9642-9648. https://doi.org/10.3390/ijms13089642
Chicago/Turabian StyleMoon, Hyung-In, and Jai-Heon Lee. 2012. "Neuroprotective Effects of Triterpene Glycosides from Glycine max against Glutamate Induced Toxicity in Primary Cultured Rat Cortical Cells" International Journal of Molecular Sciences 13, no. 8: 9642-9648. https://doi.org/10.3390/ijms13089642




