Immunomodulating Activity of Nymphaea rubra Roxb. Extracts: Activation of Rat Dendritic Cells and Improvement of the TH1 Immune Response
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction and Analysis of Nymphaea rubra Roxb. Composition
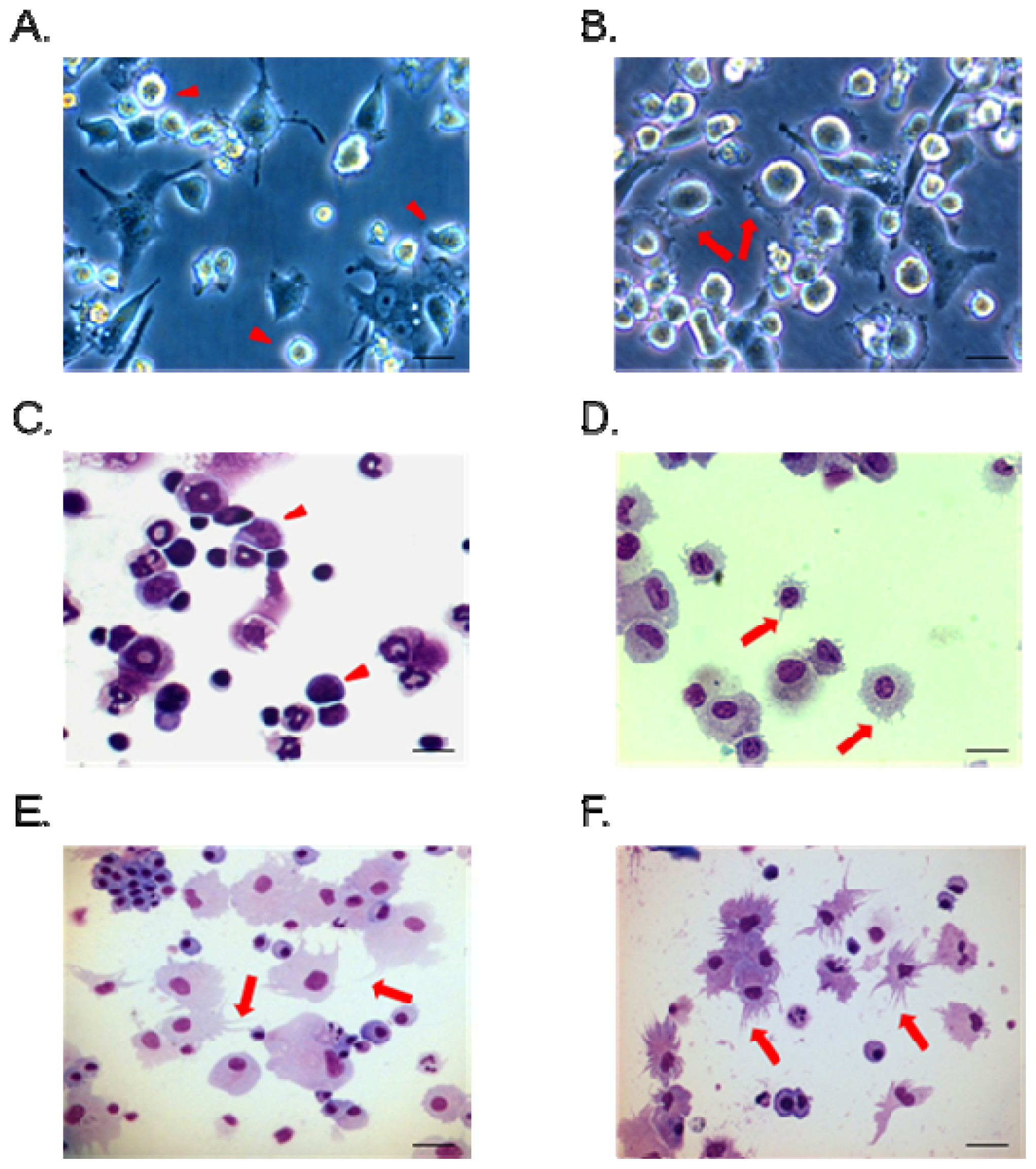
2.2. Morphological Changes and Activation of BMHC-Derived Immature Dendritic Cells (BHMC-imDCs) after NR-PS Treatment
2.3. CD11c, MHC Class II and CD80/86 Expression by Dendritic Cells after NR-PS Activation
2.4. The Endocytosis of Dendritic Cells Was Reduced after NR-PS Treatment
2.5. NR-PS Treatment of Dendritic Cells Results in Increased IL-12/IFN-γ Secretion and Reduced IL-10 Secretion
3. Experimental Section
3.1. Rats
3.2. Preparation of Crude and Hydrolyzed Nymphaea rubra Roxb. Extract
3.3. Measurement of Sugar, Protein and Polymerization Levels of the Nymphaea rubra Roxb. Extract
3.4. Isolation of Rat BMHCs
3.5. Observation of the Cell Morphology of the BMHC-imDCs
3.6. Measurement of the BMHC-imDC Surface Markers by FACS Analysis
3.7. Assessing the Endocytotic Activity of BMHC-imDCs
3.8. Enzyme-Linked Immunosorbent Assay (ELISA) for Cytokine Detection
3.9. Statistical Analysis
4. Conclusion
Acknowledgement
Abbreviations
| NR-PS | Nymphaea rubra Roxb. Polysaccharides |
| DCs | dendritic cells |
| BMHCs | bone marrow hematopoietic cells |
| BHMC-imDCs | BMHC-derived immature dendritic cells |
| DP | degree of polymerization |
| TCA | trichloroacetic acid |
| LPS | lipopolysaccharides |
| DB | degree of branching |
References
- Agnihotri, V.K.; Elsohly, H.N.; Khan, S.I.; Smillie, T.J.; Khan, I.A.; Walker, L.A. Antioxidant constituents of Nymphaea caerulea flowers. Phytochemistry 2008, 69, 2061–2066. [Google Scholar]
- Zhao, J.; Liu, T.; Ma, L.; Yan, M.; Gu, Z.; Huang, Y.; Xu, F.; Zhao, Y. Antioxidant and Preventive Effects of Extract from Nymphaea candida Flower on In vitro Immunological Liver Injury of Rat Primary Hepatocyte Cultures. Evid. Based Complement. Alternat. Med 2009. [Google Scholar] [CrossRef]
- Beckett, K.A. The Concise Encyclopedia of Garden Plants; Orbis Publishing: London, UK, 1984; p. 268. [Google Scholar]
- Rajagopal, K.; Sasikala, K. Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rats. Singapore Med. J 2008, 49, 137–141. [Google Scholar]
- Mukherjee, P.K.; Saha, K.; Das, J.; Pal, M.; Saha, B.P. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med 1997, 63, 367–369. [Google Scholar]
- Mukherjee, P.K.; Saha, K.; Pal, M.; Saha, B.P. Effect of Nelumbo nucifera rhizome extract on blood sugar level in rats. J. Ethnopharmacol 1997, 58, 207–213. [Google Scholar]
- Raja, M.K.; Sethiya, N.K.; Mishra, S.H. A comprehensive review on Nymphaea stellata: A traditionally used bitter. J. Adv. Pharm. Technol. Res 2010, 1, 311–319. [Google Scholar]
- Mukherjee, D.; Khatua, T.N.; Venkatesh, P.; Saha, B.P.; Mukherjee, P.K. Immunomodulatory potential of rhizome and seed extracts of Nelumbo nucifera Gaertn. J. Ethnopharmacol 2010, 128, 490–494. [Google Scholar]
- Sinha, S.; Mukherjee, P.K.; Mukherjee, K.; Pal, M.; Mandal, S.C.; Saha, B.P. Evaluation of antipyretic potential of Nelumbo nucifera stalk extract. Phytother. Res 2000, 14, 272–274. [Google Scholar]
- Jung, H.A.; Kim, J.E.; Chung, H.Y.; Choi, J.S. Antioxidant principles of Nelumbo nucifera stamens. Arch. Pharm. Res 2003, 26, 279–285. [Google Scholar]
- Wu, M.J.; Wang, L.; Weng, C.Y.; Yen, J.H. Antioxidant activity of methanol extract of the lotus leaf (Nelumbo nucifera Gertn.). Am. J. Chin. Med 2003, 31, 687–698. [Google Scholar]
- Sohn, D.H.; Kim, Y.C.; Oh, S.H.; Park, E.J.; Li, X.; Lee, B.H. Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine 2003, 10, 165–169. [Google Scholar]
- Bhandarkar, M.R.; Khan, A. Antihepatotoxic effect of Nymphaea stellata willd., against carbon tetrachloride-induced hepatic damage in albino rats. J. Ethnopharmacol 2004, 91, 61–64. [Google Scholar]
- Varki, A. Essentials of Glycobiology, 2nd ed; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA; p. 2009.
- Sheeler, P.; Bianchi, D.E.; Sheeler, P.C.B. Cell and Molecular Biology, 3rd ed; Wiley: New York, NY, USA; p. 1987.
- Taylor, D.; Soper, R.; Green, N.P.O.; Stout, W. Biological Science, 3rd ed; Cambridge University Press: Cambridge, UK; p. 1997.
- Fang, X.; Jiang, B.; Wang, X. Purification and partial characterization of an acidic polysaccharide with complement fixing ability from the stems of Avicennia marina. J. Biochem. Mol. Biol 2006, 39, 546–555. [Google Scholar]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev 2000, 13, 523–533. [Google Scholar]
- Kim, S.P.; Kang, M.Y.; Kim, J.H.; Nam, S.H.; Friedman, M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J. Agric. Food Chem 2011, 59, 9861–9869. [Google Scholar]
- Leung, M.Y.; Fung, K.P.; Choy, Y.M. The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacology 1997, 35, 255–263. [Google Scholar]
- Zou, Y.; Meng, J.; Chen, W.; Liu, J.; Li, X.; Li, W.; Lu, C.; Shan, F. Modulation of phenotypic and functional maturation of murine dendritic cells (DCs) by purified Achyranthes bidentata polysaccharide (ABP). Int. Immunopharmacol 2011, 11, 1103–1108. [Google Scholar]
- Chan, W.K.; Cheung, C.C.; Law, H.K.; Lau, Y.L.; Chan, G.C. Ganoderma lucidum polysaccharides can induce human monocytic leukemia cells into dendritic cells with immuno-stimulatory function. J. Hematol. Oncol 2008, 1, 9. [Google Scholar]
- Bimczok, D.; Wrenger, J.; Schirrmann, T.; Rothkotter, H.J.; Wray, V.; Rau, U. Short chain regioselectively hydrolyzed scleroglucans induce maturation of porcine dendritic cells. Appl. Microbiol. Biotechnol 2009, 82, 321–331. [Google Scholar]
- Zanoni, I.; Ostuni, R.; Capuano, G.; Collini, M.; Caccia, M.; Ronchi, A.E.; Rocchetti, M.; Mingozzi, F.; Foti, M.; Chirico, G.; et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 2009, 460, 264–268. [Google Scholar]
- Granucci, F.; Zanoni, I. The dendritic cell life cycle. Cell Cycle 2009, 8, 3816–3821. [Google Scholar]
- Lanzavecchia, A. Dendritic cell maturation and generation of immune responses. Haematologica 1999, 84, 23–25. [Google Scholar]
- Sheu, S.; Lai, M. Composition analysis and immuno-modulatory effect of okra (Abelmoschus esculentus L.) extract. Food Chemistry 2012, 134, 1906–1911. [Google Scholar]
- Dong, Q.; Yao, J.; Yang, X.T.; Fang, J.N. Structural characterization of a water-soluble β-d-glucan from fruiting bodies of Agaricus blazei Murr. Carbohydr. Res 2002, 337, 1417–1421. [Google Scholar]
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-d-glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohyd. Polym. 1995, 28, 3–14. [Google Scholar]
- Ohno, N.; Furukawa, M.; Miura, N.N.; Adachi, Y.; Moto, M.; Yadomae, T. Antitumor β-Glucan from the Cultured Fruit Body of Agaricus blazei. Biol. Pharm. Bull 2001, 24, 820–828. [Google Scholar]
- Fina, D.; Sarra, M.; Fantini, M.C.; Rizzo, A.; Caruso, R.; Caprioli, F.; Stolfi, C.; Cardolini, I.; Dottori, M.; Boirivant, M.; et al. Regulation of gut inflammation and Th17 cell response by interleukin-21. Gastroenterology 2008, 134, 1038–1048. [Google Scholar]
- Istrate, C.; Douagi, I.; Charpilienne, A.; McInerney, G.M.; Hidmark, A.; Johansen, K.; Larsson, M.; Magnusson, K.E.; Poncet, D.; Svensson, L.; et al. Bone marrow dendritic cells internalize live RF-81 bovine rotavirus and rotavirus-like particles (RF 2/6-GFP-VLP and RF 8*2/6/7-VLP) but are only activated by live bovine rotavirus. Scand. J. Immunol 2007, 65, 494–502. [Google Scholar]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med 1992, 176, 1693–1702. [Google Scholar]
- Talmor, M.; Mirza, A.; Turley, S.; Mellman, I.; Hoffman, L.A.; Steinman, R.M. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur. J. Immunol 1998, 28, 811–817. [Google Scholar]
- Acosta, C.; Davies, A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J. Neurosci. Res 2008, 86, 1077–1086. [Google Scholar]
- Zhao, L.; Kaneko, T.; Okiji, T.; Takagi, M.; Suda, H. Immunoelectron microscopic analysis of CD11c-positive dendritic cells in the periapical region of the periodontal ligament of rat molars. J. Endod 2006, 32, 1164–1167. [Google Scholar]
- Kikuchi, T.; Ohno, N.; Ohno, T. Maturation of dendritic cells induced by Candida β-d-glucan. Int. Immunopharmacol 2002, 2, 1503–1508. [Google Scholar]
- Kato, M.; Neil, T.K.; Fearnley, D.B.; McLellan, A.D.; Vuckovic, S.; Hart, D.N. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int. Immunol 2000, 12, 1511–1519. [Google Scholar]
- Menges, M.; Baumeister, T.; Rossner, S.; Stoitzner, P.; Romani, N.; Gessner, A.; Lutz, M.B. IL-4 supports the generation of a dendritic cell subset from murine bone marrow with altered endocytosis capacity. J. Leukoc. Biol 2005, 77, 535–543. [Google Scholar]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol 2000, 18, 767–811. [Google Scholar]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar]
- Keller, R. Dendritic cells: Their significance in health and disease. Immunol. Lett 2001, 78, 113–122. [Google Scholar]
- Moser, M.; Murphy, K.M. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol 2000, 1, 199–205. [Google Scholar]
- Stumbles, P.A.; Thomas, J.A.; Pimm, C.L.; Lee, P.T.; Venaille, T.J.; Proksch, S.; Holt, P.G. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med 1998, 188, 2019–2031. [Google Scholar]
- Trinchieri, G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol 1995, 13, 251–276. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem 1959, 31, 426–428. [Google Scholar]
- Shlieout, G.; Arnold, K.; Muller, G. Powder and mechanical properties of microcrystalline cellulose with different degrees of polymerization. AAPS PharmSciTech 2002, 3, E11. [Google Scholar]


| Extract | Yield | Total sugar | Reducing sugar | Protein |
|---|---|---|---|---|
| Crude | 1.43 | 55.86 ± 0.80 a | 2.64± 0.34 a | 7.84 ± 0.63 a |
| Hydrolyzed | 42.00 | 51.30 ± 0.98 a | 2.45 ± 0.92 a | 5.28 ± 0.28 b |
| Groups | Percentages of cells with a positive cell surface antigen reaction * | |
|---|---|---|
| CD 80/86 ** | MHC II ** | |
| Control | 65.45 ± 0.97 | 34.87 ± 1.96 |
| LPS (μg/mL) | ||
| 1 | 85.76 ± 3.06 | 73.20 ± 6.16 |
| NR-PS (μg/mL) | ||
| 3.125 | 84.49 ± 10.86 | 45.06 ± 8.11 |
| 6.25 | 84.80 ± 7.94 | 44.88 ± 5.11 |
| 12.5 | 85.68 ± 18.66 | 49.18 ± 10.41 |
| 25 | 87.16 ± 8.49 | 52.01 ± 10.11 |
| 50 | 84.44 ± 6.35 | 49.27 ± 5.03 |
| 100 | 84.56 ± 7.62 | 50.27 ± 5.24 |
| Groups | Mean fluorescence intensity of dendritic cells * | |
|---|---|---|
| CD 80/86 ** | MHC II ** | |
| Control | 24.60 ± 3.19 | 46.55 ± 4.97 |
| LPS (μg/mL) | ||
| 1 | 49.00 ± 9.18 | 57.62 ± 1.87 |
| NR-PS (μg/mL) | ||
| 3.125 | 47.23 ± 3.72 | 51.09 ± 4.73 |
| 6.25 | 42.73 ± 4.20 | 51.94 ± 2.89 |
| 12.5 | 45.69 ± 3.58 | 55.62 ± 8.21 |
| 25 | 48.48 ± 4.26 | 60.74 ± 10.06 |
| 50 | 38.03 ± 6.27 | 57.30 ± 4.76 |
| 100 | 43.70 ± 7.61 | 57.70 ± 2.68 |
| Groups | Uptake of FITC-dextran * |
|---|---|
| Mean fluorescence intensity ** | |
| Control 37 °C | 261.67 ± 47.26 |
| LPS (μg/mL) | |
| 1 | 121.60 ± 31.05 |
| NR-PS (μg/mL) | |
| 3.125 | 227.87 ± 35.88 |
| 6.25 | 192.47 ± 52.47 |
| 12.5 | 176.50 ± 28.52 |
| 25 | 167.94 ± 60.59 |
| 50 | 199.41 ± 24.52 |
| 100 | 211.49 ± 33.65 |
| Groups | Level of cytokine * (pg/mL) | ||
|---|---|---|---|
| IL-12 ** | IL-10 ** | IFN-γ ** | |
| Control | 102.09 ± 10.16 | 30.75 ± 3.35 | 11.76 ± 0.11 |
| NR-PS (μg/mL) | |||
| 3.125 | 158.59 ± 37.85 | 23.20 ± 8.47 | 15.40 ± 1.52 |
| 6.25 | 221.40 ± 11.90 | 22.48 ± 5.44 | 15.33 ± 2.62 |
| 12.5 | 220.65 ± 17.09 | 18.16 ± 4.17 | 15.96 ± 2.66 |
| 25 | 258.78 ± 25.26 | 15.37 ± 2.35 | 15.51 ± 1.66 |
| 50 | 254.62 ± 36.22 | 14.83 ± 3.80 | 15.01 ± 1.47 |
| 100 | 194.48 ± 27.61 | 21.22 ± 2.11 | 15.67 ± 1.24 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, J.-H.; Lee, S.-Y.; Lien, Y.-Y.; Lee, M.-S.; Sheu, S.-C. Immunomodulating Activity of Nymphaea rubra Roxb. Extracts: Activation of Rat Dendritic Cells and Improvement of the TH1 Immune Response. Int. J. Mol. Sci. 2012, 13, 10722-10735. https://doi.org/10.3390/ijms130910722
Cheng J-H, Lee S-Y, Lien Y-Y, Lee M-S, Sheu S-C. Immunomodulating Activity of Nymphaea rubra Roxb. Extracts: Activation of Rat Dendritic Cells and Improvement of the TH1 Immune Response. International Journal of Molecular Sciences. 2012; 13(9):10722-10735. https://doi.org/10.3390/ijms130910722
Chicago/Turabian StyleCheng, Jai-Hong, Shau-Yu Lee, Yi-Yang Lien, Meng-Shiou Lee, and Shyang-Chwen Sheu. 2012. "Immunomodulating Activity of Nymphaea rubra Roxb. Extracts: Activation of Rat Dendritic Cells and Improvement of the TH1 Immune Response" International Journal of Molecular Sciences 13, no. 9: 10722-10735. https://doi.org/10.3390/ijms130910722




