Combined Treatment with an Oncolytic Adenovirus and Antitumor Activity of Vincristine against Retinoblastoma Cells
Abstract
:1. Introduction
2. Results and Discussion
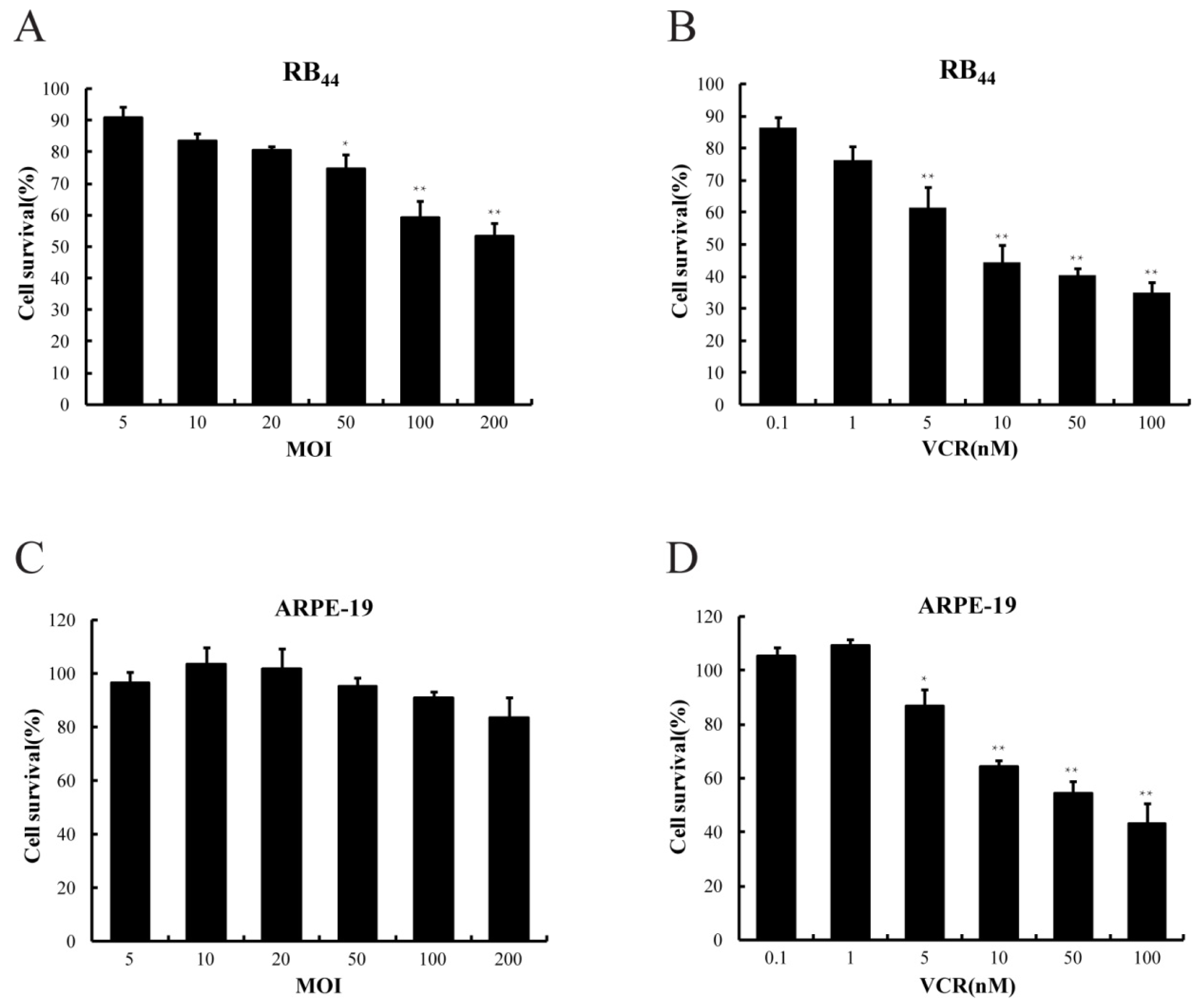
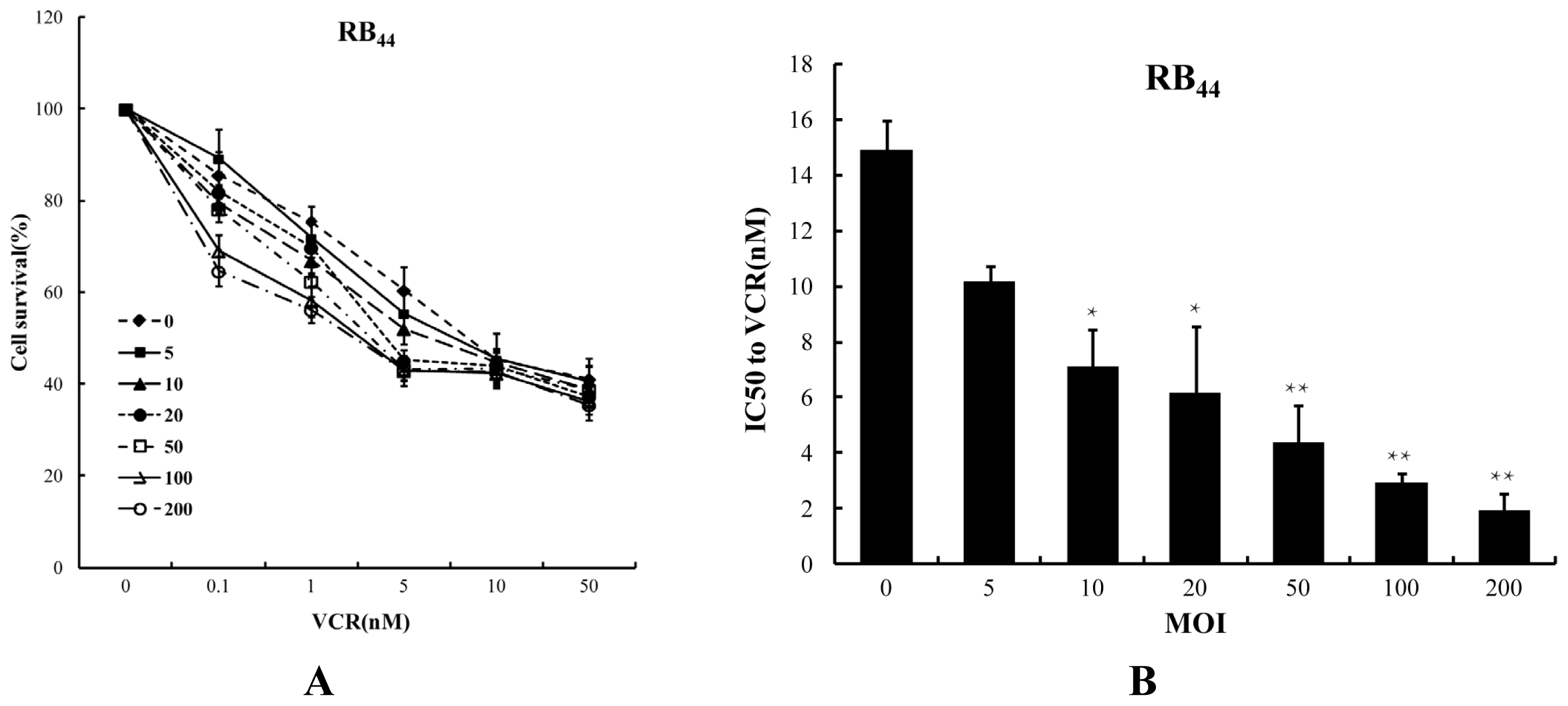
2.1. Cytotoxicity of HXO-RB44 Cells by the Combined Treatment of SG600 and VCR
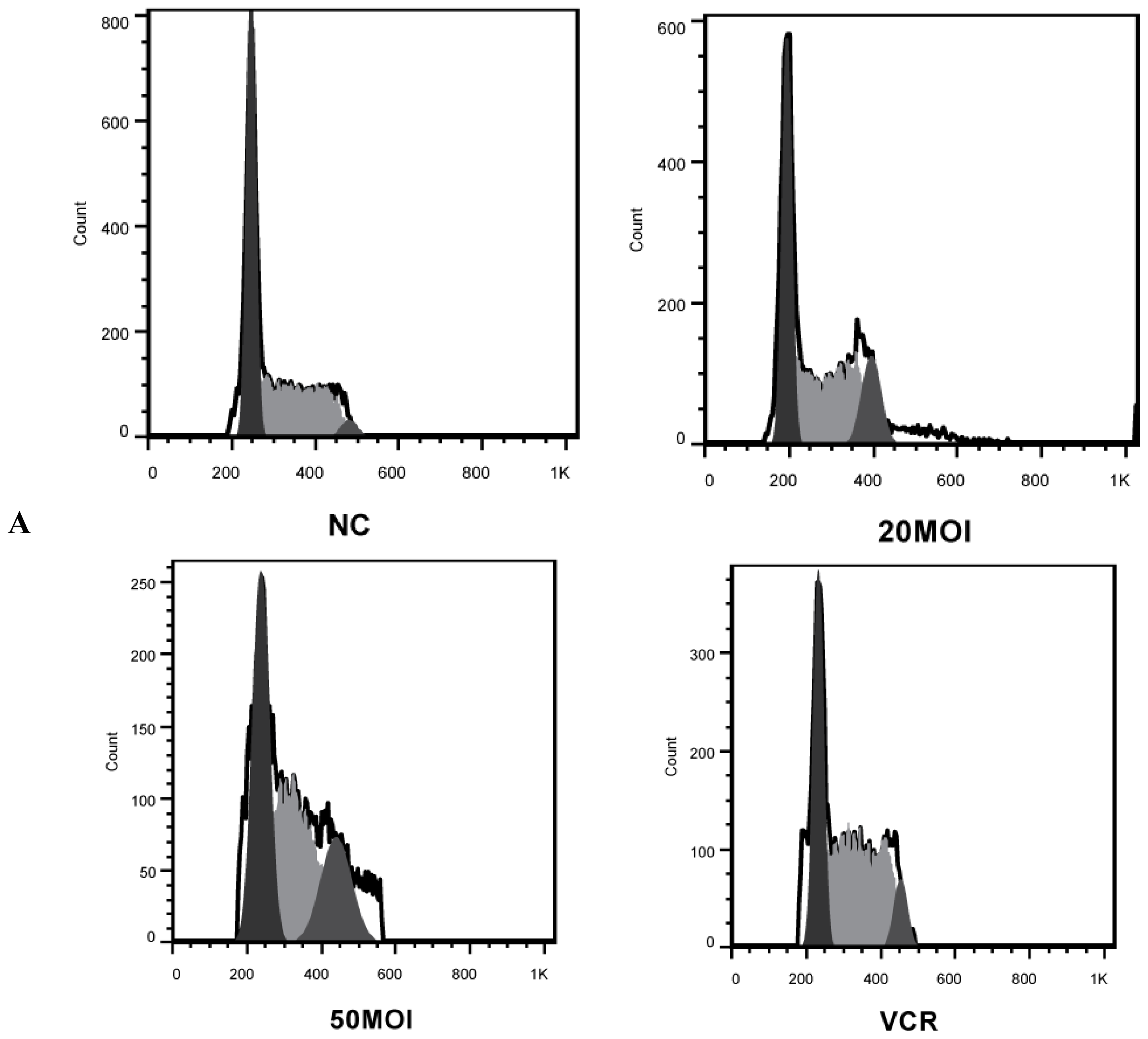
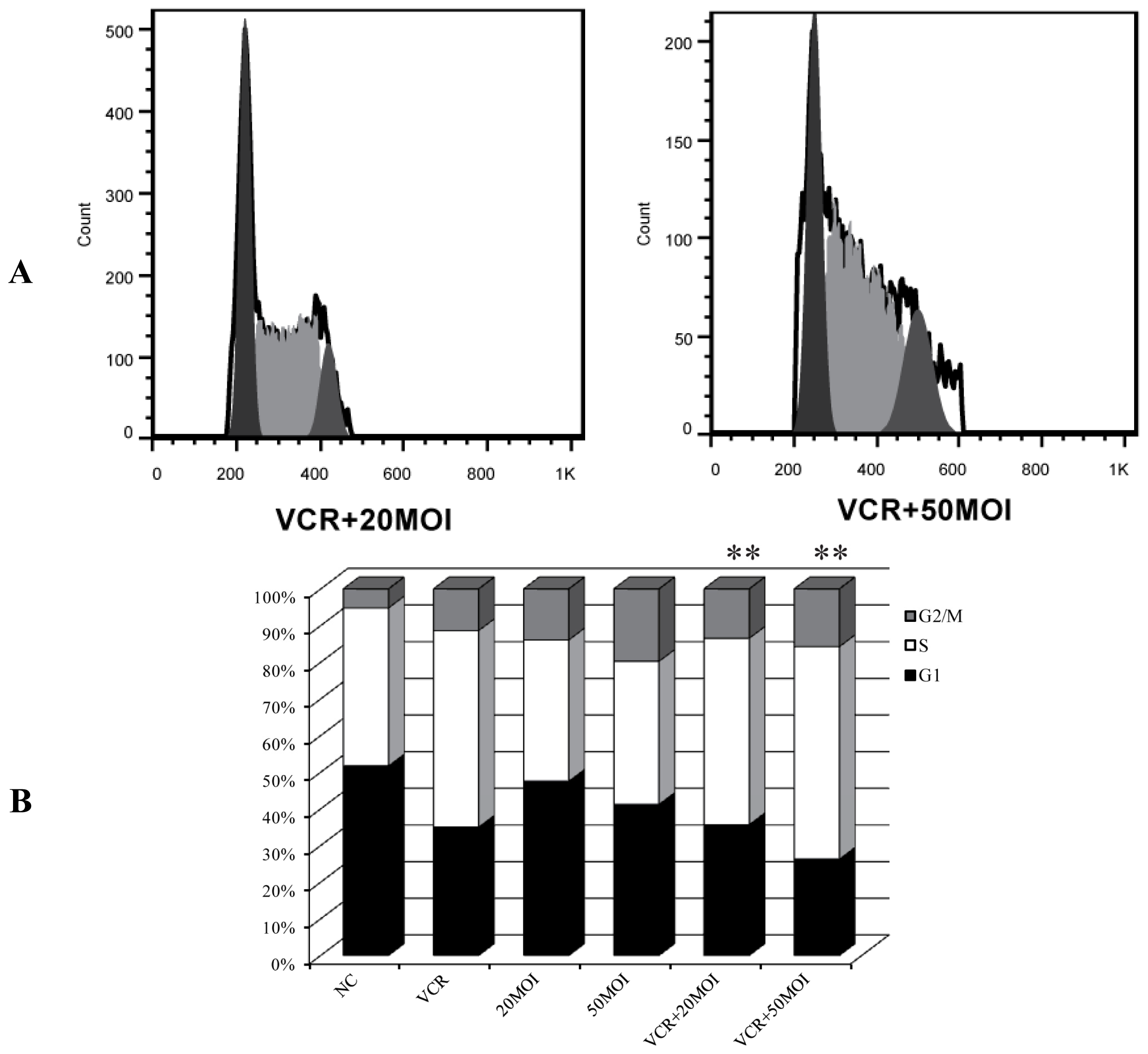
2.2. Alteration of Cell Cycle Distribution by Combination Treatment
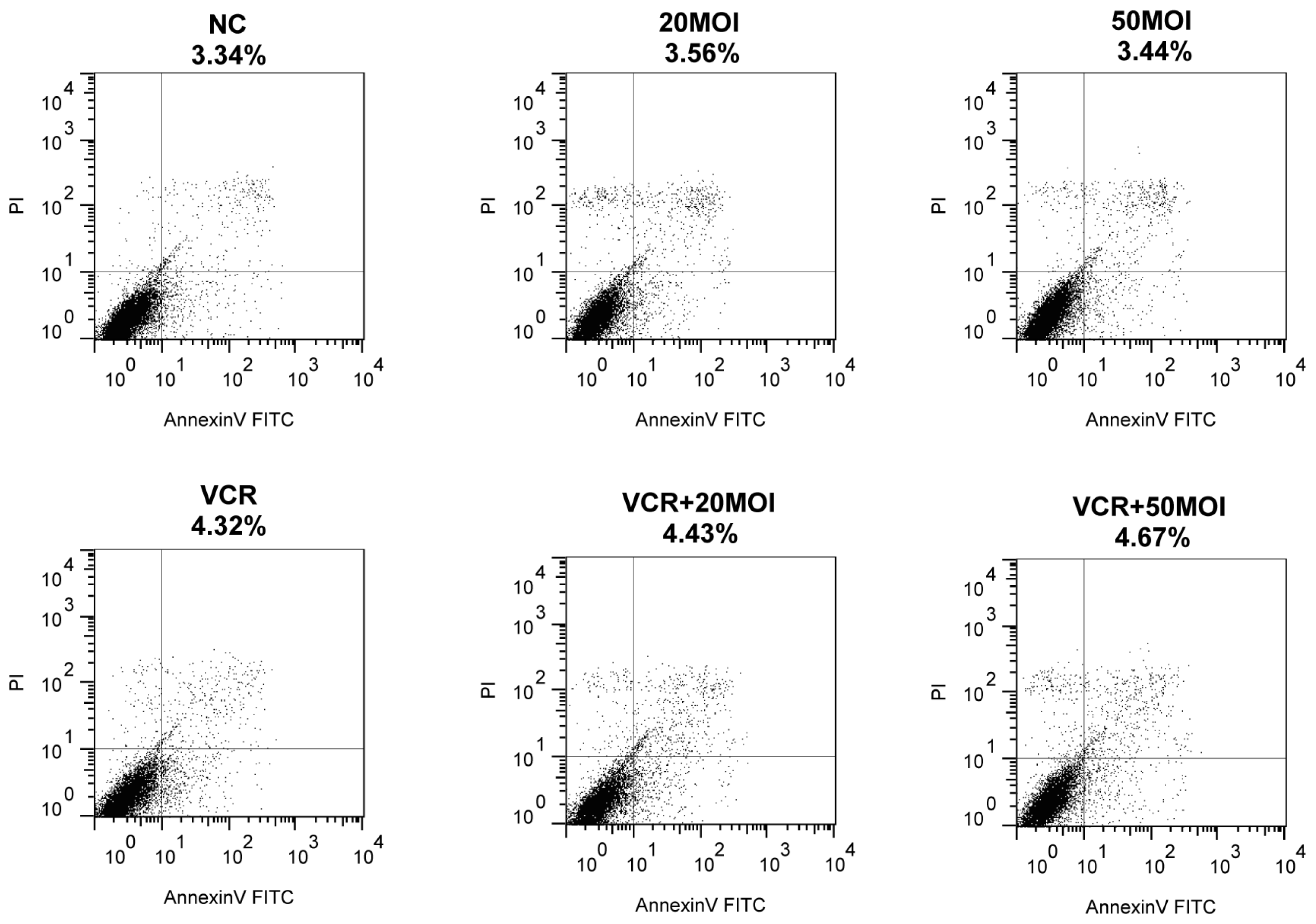
2.3. Function of Apoptosis by Combined SG600 and VCR
2.4. P-Akt Protein Expression in HXO-RB44 Cells Following Combined Treatment with SG600 and VCR
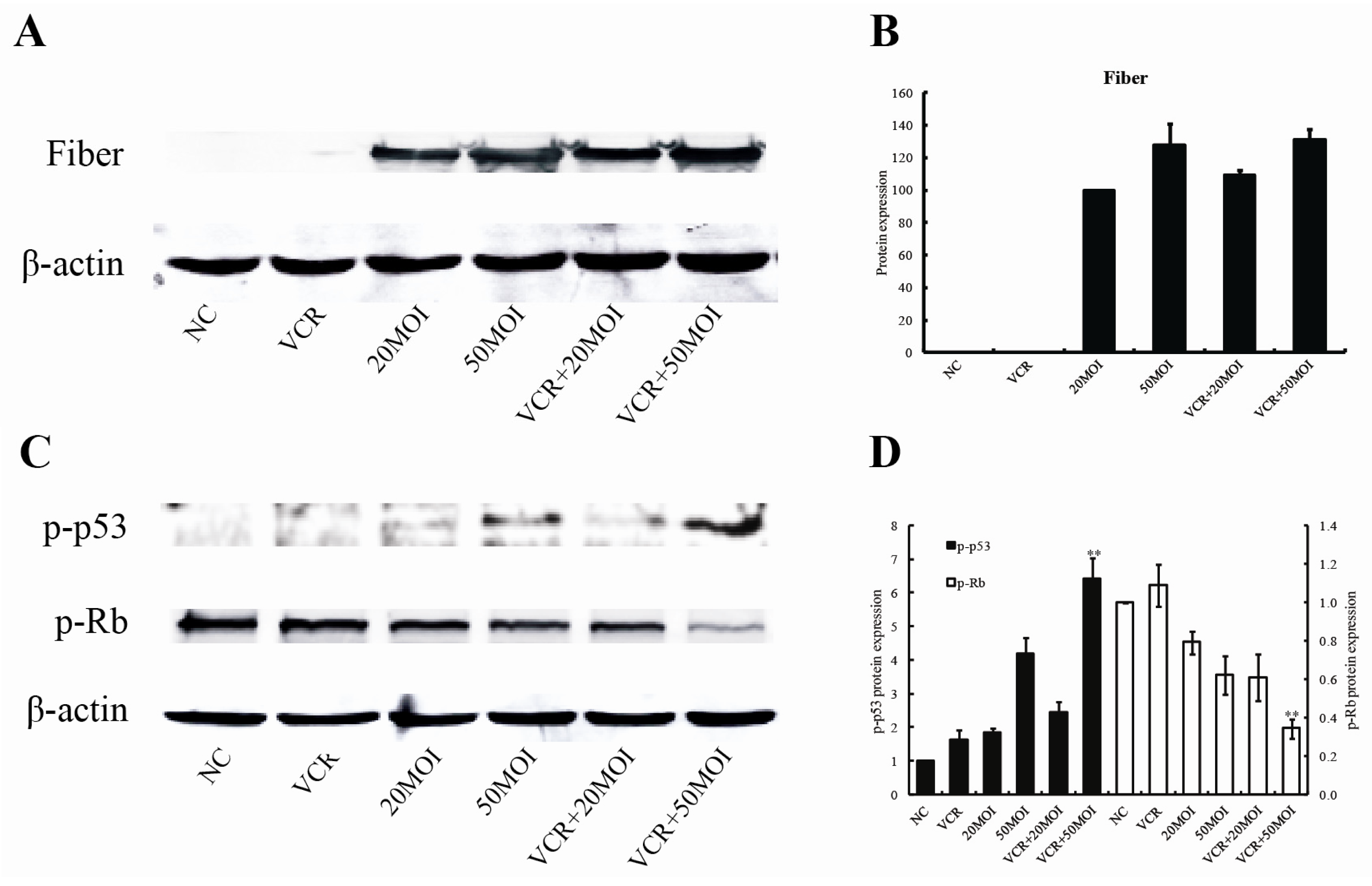
2.5. Effect of SG600 Replication by Combined SG600 and VCR
2.6. Altered p-p53 and p-Rb Protein Expression Following Combined Treatment with SG600 and VCR
2.7. Discussion
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Oncolytic Adenoviruses
3.3. Cell Lines and Cell Culture
3.4. Cell Viability Assay
3.5. Cell Cycle
3.6. Apoptotic Analysis
3.7. Western Blot Analysis for Akt, p-Akt, Fiber, p-p53 and p-Rb Protein
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Devesa, S.S. The incidence of retinoblastoma. Am. J. Ophthalmol 1975, 80, 263–265. [Google Scholar]
- Murphree, A.L.; Villablanca, J.G.; Deegan, W.F., III; Sato, J.K.; Malogolowkin, M.; Fisher, A.; Parker, R.; Reed, E.; Gomer, C.J. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch. Ophthalmol. 1996, 114, 1348–1356. [Google Scholar]
- Abramson, D.H.; Frank, C.M.; Dunkel, I.J. A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma. Ophthalmology 1999, 106, 1947–1950. [Google Scholar]
- Chan, H.S.; Canton, M.D.; Gallie, B.L. Chemosensitivity and multidrug resistance to antineoplastic drugs in retinoblastoma cell lines. Anticancer Res 1989, 9, 469–474. [Google Scholar]
- Chan, H.S.; Thorner, P.S.; Haddad, G.; Gallie, B.L. Multidrug-resistant phenotype in retinoblastoma correlates with P-glycoprotein expression. Ophthalmology 1991, 98, 1425–1431. [Google Scholar]
- Wilson, M.W.; Fraga, C.H.; Rodriguez-Galindo, C.; Hagedorn, N.; Leggas, M.L.; Stewart, C. Expression of the multi-drug resistance proteins and the pregnane X receptor in treated and untreated retinoblastoma. Curr. Eye Res 2009, 34, 386–394. [Google Scholar]
- Wilson, M.W.; Fraga, C.H.; Fuller, C.E.; Rodriguez-Galindo, C.; Mancini, J.; Hagedorn, N.; Leggas, M.L.; Stewart, C.F. Immunohistochemical detection of multidrug-resistant protein expression in retinoblastoma treated by primary enucleation. Investig. Ophthalmol. Vis. Sci 2006, 47, 1269–1273. [Google Scholar]
- Cun, B.; Song, X.; Jia, R.; Zhao, X.; Wang, H.; Ge, S.; Fan, X. Combination of oncolytic adenovirus and dacarbazine enhances antitumor ability against uveal melanoma cells via cell cycle block. Cancer Biol. Ther 2012, 13, 77–84. [Google Scholar]
- Song, X.; Zhou, Y.; Jia, R.; Xu, X.; Wang, H.; Hu, J.; Ge, S.; Fan, X. Inhibition of retinoblastoma in vitro and in vivo with conditionally replicating oncolytic adenovirus H101. Investig. Ophthalmol. Vis. Sci 2010, 51, 2626–2635. [Google Scholar]
- Zhang, H.; Wang, H.; Zhang, J.; Qian, G.; Niu, B.; Fan, X.; Lu, J.; Hoffman, A.R.; Hu, J.F.; Ge, S. Enhanced therapeutic efficacy by simultaneously targeting two genetic defects in tumors. Mol. Ther. J. Am. Soc. Gene Ther 2009, 17, 57–64. [Google Scholar]
- Gomez-Manzano, C.; Alonso, M.M.; Yung, W.K.; McCormick, F.; Curiel, D.T.; Lang, F.F.; Jiang, H.; Bekele, B.N.; Zhou, X.; Alemany, R.; et al. Delta-24 increases the expression and activity of topoisomerase I and enhances the antiglioma effect of irinotecan. Clin. Cancer Res 2006, 12, 556–562. [Google Scholar]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.X.; Levin, V.A.; Yung, W.K.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar]
- Wang, X.; Su, C.; Cao, H.; Li, K.; Chen, J.; Jiang, L.; Zhang, Q.; Wu, X.; Jia, X.; Liu, Y.; et al. A novel triple-regulated oncolytic adenovirus carrying p53 gene exerts potent antitumor efficacy on common human solid cancers. Mol. Cancer Ther 2008, 7, 1598–1603. [Google Scholar]
- Friend, S.H.; Bernards, R.; Rogelj, S.; Weinberg, R.A.; Rapaport, J.M.; Albert, D.M.; Dryja, T.P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 1986, 323, 643–646. [Google Scholar]
- Laurie, N.A.; Donovan, S.L.; Shih, C.S.; Zhang, J.; Mills, N.; Fuller, C.; Teunisse, A.; Lam, S.; Ramos, Y.; Mohan, A.; et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006, 444, 61–66. [Google Scholar]
- Laurie, N.A.; Shih, C.S.; Dyer, M.A. Targeting MDM2 and MDMX in retinoblastoma. Curr. Cancer Drug Targets 2007, 7, 689–695. [Google Scholar]
- Marine, J.C.; Dyer, M.A.; Jochemsen, A.G. MDMX: From bench to bedside. J. Cell Sci 2007, 120, 371–378. [Google Scholar]
- Ottolino-Perry, K.; Diallo, J.S.; Lichty, B.D.; Bell, J.C.; McCart, J.A. Intelligent design: Combination therapy with oncolytic viruses. Mol. Ther. J. Am. Soc. Gene Ther 2010, 18, 251–263. [Google Scholar]
- Cheema, T.A.; Kanai, R.; Kim, G.W.; Wakimoto, H.; Passer, B.; Rabkin, S.D.; Martuza, R.L. Enhanced antitumor efficacy of low-dose Etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clin. Cancer Res 2011, 17, 7383–7393. [Google Scholar]
- Hoffmann, D.; Bayer, W.; Wildner, O. Local and distant immune-mediated control of colon cancer growth with fusogenic membrane glycoproteins in combination with viral oncolysis. Hum. Gene Ther 2007, 18, 435–450. [Google Scholar]
- Cinatl, J., Jr; Cinatl, J.; Michaelis, M.; Kabickova, H.; Kotchetkov, R.; Vogel, J.U.; Doerr, H.W.; Klingebiel, T.; Driever, P.H. Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res. 2003, 63, 1508–1514. [Google Scholar]
- De Potter, P. Current treatment of retinoblastoma. Curr. Opin. Ophthalmol 2002, 13, 331–336. [Google Scholar]
- Balwierz, W.; Pietrys, D.; Romanowska-Dixon, B.; Kobylarz, J.; Pawinska-Wasikowska, K.; Moryl-Bujakowska, A. Results of combined chemotherapy and local ophtalmic therapy for intraocular retinoblastoma. Przegl. Lek 2010, 67, 404–408. [Google Scholar]
- Qian, J.; Xue, K.; Gao, Y.J.; Yuan, Y.F.; Shan, H.D.; Bi, Y.W. Clinical therapeutic efficiency of chemoreduction and local therapy for children with retinoblastoma (in chinese). Zhonghua Yanke Zazhi 2010, 46, 312–316. [Google Scholar]
- Kim, H.; Lee, J.W.; Kang, H.J.; Park, H.J.; Kim, Y.Y.; Shin, H.Y.; Yu, Y.S.; Kim, I.H.; Ahn, H.S. Clinical results of chemotherapy based treatment in retinoblastoma patients: A single center experience. Cancer Res. Treat 2008, 40, 164–171. [Google Scholar]
- Shields, C.L.; Shields, J.A. Basic understanding of current classification and management of retinoblastoma. Curr. Opin. Ophthalmol 2006, 17, 228–234. [Google Scholar]
- Turaka, K.; Shields, C.L.; Meadows, A.T.; Leahey, A. Second malignant neoplasms following chemoreduction with carboplatin, etoposide, and vincristine in 245 patients with intraocular retinoblastoma. Pediatr. Blood Cancer 2011, 59, 121–125. [Google Scholar]
- Ji, X.; Zhang, J.; Cheng, L.; Wei, F.; Li, H.; Liu, X.; Chen, X.; Li, C.; Wang, Y.; Huang, Q. Oncolytic adenovirus delivering herpes simplex virus thymidine kinase suicide gene reduces the growth of human retinoblastoma in an in vivo mouse model. Exp. Eye Res 2009, 89, 193–199. [Google Scholar]
- Gallie, B.L.; Dunn, J.M.; Chan, H.S.; Hamel, P.A.; Phillips, R.A. The genetics of retinoblastoma. Relevance to the patient. Pediatr. Clin. North Am 1991, 38, 299–315. [Google Scholar]
- Abramson, D.H.; Schefler, A.C. Update on retinoblastoma. Retina 2004, 24, 828–848. [Google Scholar]
- Nevins, J.R. E2F: A link between the Rb tumor suppressor protein and viral oncoproteins. Science 1992, 258, 424–429. [Google Scholar]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/Akt pathway for cancer drug discovery. Nat. Rev. Drug Discov 2005, 4, 988–1004. [Google Scholar]
- Gu, P.; Su, Y.; Guo, S.; Teng, L.; Xu, Y.; Qi, J.; Gong, H.; Cai, Y. Over-expression of COX-2 induces human ovarian cancer cells (CAOV-3) viability, migration and proliferation in association with PI3-k/Akt activation. Cancer Investig 2008, 26, 822–829. [Google Scholar]
- Zhang, H.; Li, B.; Bai, S.W.; Wang, H.J. Constitutively active Akt contributes to vincristine resistance in human retinoblastoma cells. Cancer Investig 2010, 28, 156–165. [Google Scholar]
- Bernt, K.M.; Steinwaerder, D.S.; Ni, S.; Li, Z.Y.; Roffler, S.R.; Lieber, A. Enzyme-activated prodrug therapy enhances tumor-specific replication of adenovirus vectors. Cancer Res 2002, 62, 6089–6098. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, X.; Wang, H.; Jia, R.; Cun, B.; Zhao, X.; Zhou, Y.; Xu, X.; Qian, G.; Ge, S.; Fan, X. Combined Treatment with an Oncolytic Adenovirus and Antitumor Activity of Vincristine against Retinoblastoma Cells. Int. J. Mol. Sci. 2012, 13, 10736-10749. https://doi.org/10.3390/ijms130910736
Song X, Wang H, Jia R, Cun B, Zhao X, Zhou Y, Xu X, Qian G, Ge S, Fan X. Combined Treatment with an Oncolytic Adenovirus and Antitumor Activity of Vincristine against Retinoblastoma Cells. International Journal of Molecular Sciences. 2012; 13(9):10736-10749. https://doi.org/10.3390/ijms130910736
Chicago/Turabian StyleSong, Xin, Haibo Wang, Renbing Jia, Biyun Cun, Xiaoping Zhao, Yixiong Zhou, Xiaofang Xu, Guanxiang Qian, Shengfang Ge, and Xianqun Fan. 2012. "Combined Treatment with an Oncolytic Adenovirus and Antitumor Activity of Vincristine against Retinoblastoma Cells" International Journal of Molecular Sciences 13, no. 9: 10736-10749. https://doi.org/10.3390/ijms130910736



