Studies on the Interactions of Copper and Zinc Ions with β-Amyloid Peptides by a Surface Plasmon Resonance Biosensor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Aβ Immobilization
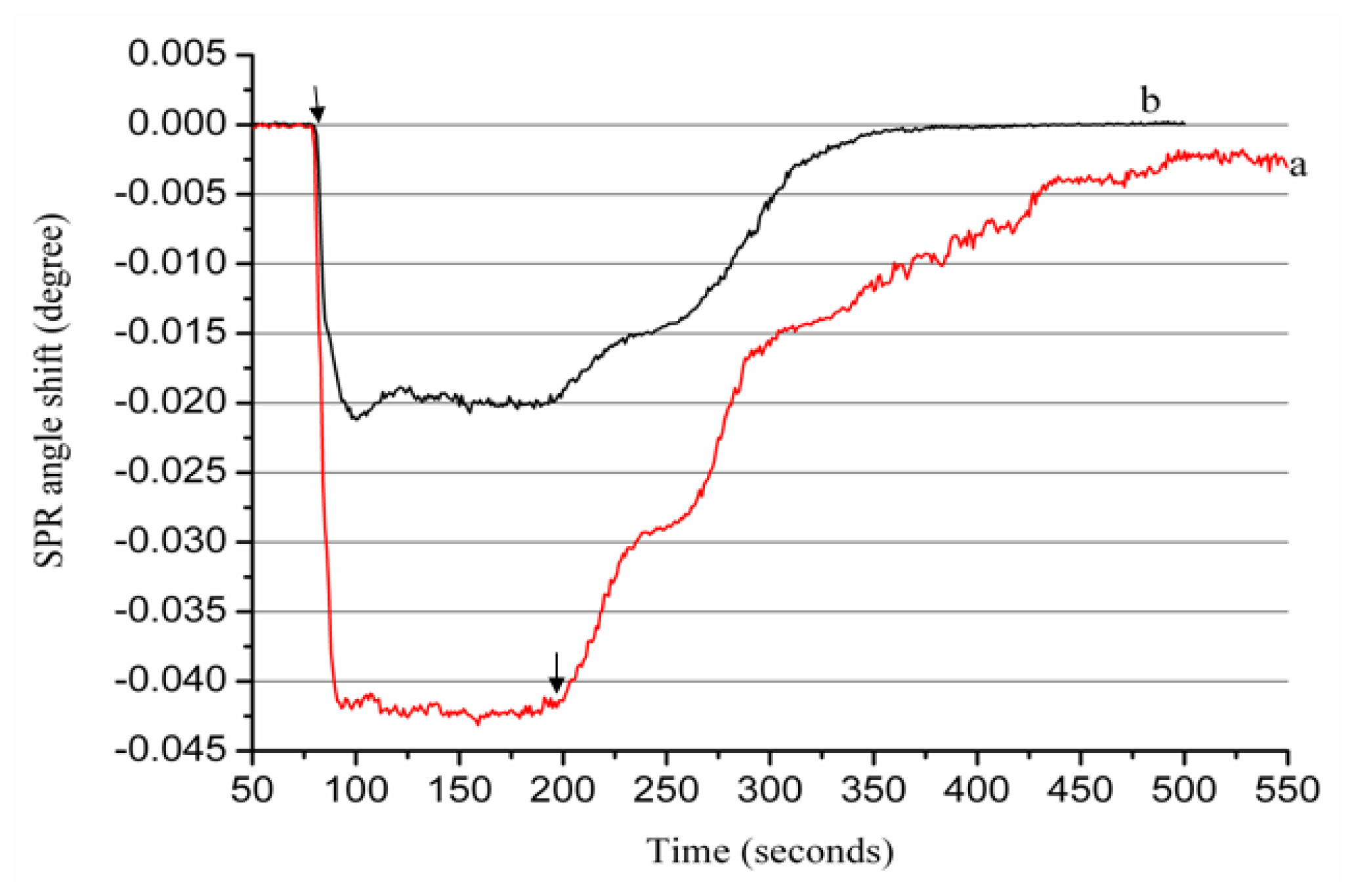
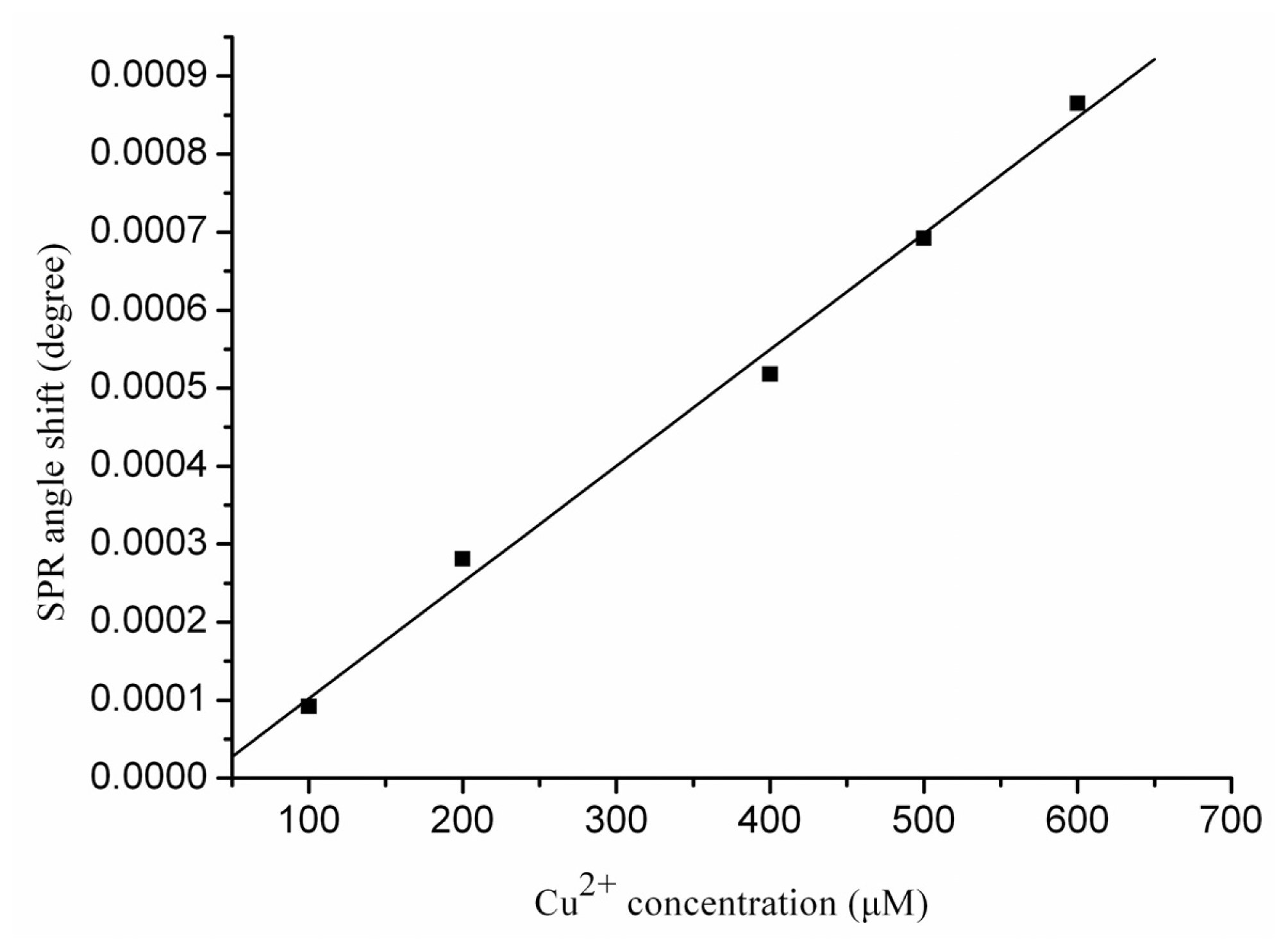
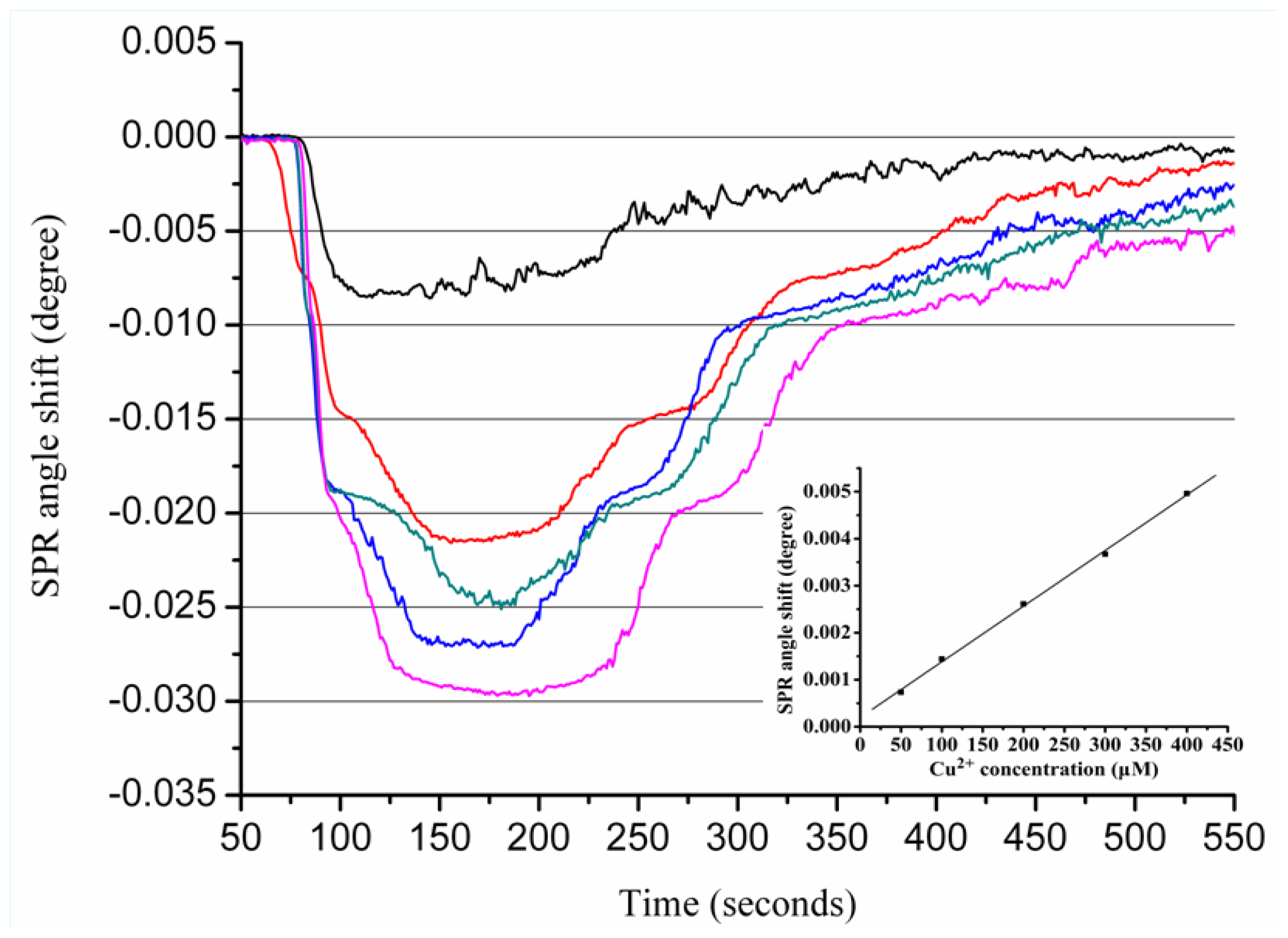
2.2. Real-Time Determination of Aβ1–28 Binding Zn2+ and Cu2+ by SPR
2.3. Real-Time Determination of Aβ1–42 Binding to Zn2+ and Cu2+ by SPR
3. Experimental Section
3.1. Chemicals
3.2. Preparation of Fresh Aβ Solution
3.3. Gold Film Preparation
3.4. Aβ Peptide Immobilization
3.5. SPR Apparatus
4. Conclusions
Acknowledgments
References
- Roychaudhuri, R.; Yang, M.; Hoshi, M.M.; Teplow, D.B. Amyloid β-protein assembly and Alzheimer disease. J. Biol. Chem 2009, 284, 4749–4753. [Google Scholar]
- Burdick, D.; Soreghan, B.; Kwon, M.; Kosmoski, J.; Knauer, M.; Henschen, A.; Yates, J.; Cotman, C.; Glabe, C. Assembly and aggregation properties of synthetic Alzheimer’s A4/β amyloid peptide analogs. J. Biol. Chem 1992, 267, 546–554. [Google Scholar]
- Fraser, P.E.; Nguyen, J.T.; Surewicz, W.K.; Kirschner, D.A. pH-dependent structural transitions of Alzheimer amyloid peptides. Biophys. J 1991, 60, 1190–1201. [Google Scholar]
- McLaurin, J.; Franklin, T.; Chakrabartty, A.; Fraser, P.E. Phosphatidylinositol and inositol involvement in Alzheimer amyloid-β fibril growth and arrest. J. Mol. Biol 1998, 278, 183–194. [Google Scholar]
- Yamaguchi, T.; Matsuzaki, K.; Hoshino, M. Transient formation of intermediate conformational states of amyloid-β peptide revealed by heteronuclear magnetic resonance spectroscopy. FEBS Lett 2011, 585, 1097–1102. [Google Scholar] [Green Version]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; d Paradis, M.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar]
- Miura, T.; Suzuki, K.; Kohata, N.; Takeuchi, H. Metal binding modes of Alzheimer’s amyloid β-peptide in insoluble aggregates and soluble complexes. Biochemistry 2000, 39, 7024–7031. [Google Scholar]
- Kozin, S.A.; Zirah, S.; Rebuffat, S.; Hoa, G.H.; Debey, P. Zinc binding to Alzheimer’s Aβ(1–16) peptide results in stable soluble complex. Biochem. Biophys. Res. Commun 2001, 285, 959–964. [Google Scholar]
- Chen, T.; Wang, X.; He, Y.; Zhang, C.; Wu, Z.; Liao, K.; Wang, J.; Guo, Z. Effects of cyclen and cyclam on zinc(II)- and copper(II)-induced amyloid β-peptide aggregation and neurotoxicity. Inorg. Chem 2009, 48, 5801–5809. [Google Scholar]
- Sarell, C.J.; Syme, C.D.; Rigby, S.E.; Viles, J.H. Copper(II) binding to amyloid-beta fibrils of Alzheimer’s disease reveals a picomolar affinity: Stoichiometry and coordination geometry are independent of Aβ oligomeric form. Biochemistry 2009, 48, 4388–4402. [Google Scholar]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci 1998, 158, 47–52. [Google Scholar]
- Atwood, C.S.; Moir, R.D.; Huang, X.; Scarpa, R.C.; Bacarra, N.M.; Romano, D.M.; Hartshorn, M.A.; Tanzi, R.E.; Bush, A.I. Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem 1998, 273, 12817–12826. [Google Scholar]
- Zou, J.; Kajita, K.; Sugimoto, N. Cu2+ inhibits the aggregation of amyloid β-peptide(1–42) in vitro. Angew. Chem. Int. Ed 2001, 40, 2274–2277. [Google Scholar]
- Raman, B.; Ban, T.; Yamaguchi, K.; Sakai, M.; Kawai, T.; Naiki, H.; Goto, Y. Metal ion-dependent effects of clioquinol on the fibril growth of an amyloid β peptide. J. Biol. Chem 2005, 280, 16157–16162. [Google Scholar]
- Huang, X.; Atwood, C.S.; Hartshorn, M.A.; Multhaup, G.; Goldstein, L.E.; Scarpa, R.C.; Cuajungco, M.P.; Gray, D.N.; Lim, J.; Moir, R.D.; et al. The Aβ peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 1999, 38, 7609–7616. [Google Scholar]
- Kowalik-Jankowska, T.; Ruta, M.; Wisniewska, K.; Lankiewicz, L.; Dyba, M. Products of Cu(II)-catalyzed oxidation in the presence of hydrogen peroxide of the 1–10, 1–16 fragments of human and mouse β-amyloid peptide. J. Inorg. Biochem 2004, 98, 940–950. [Google Scholar]
- Ali, F.E.; Separovic, F.; Barrow, C.J.; Cherny, R.A.; Fraser, F.; Bush, A.I.; Masters, C.L.; Barnham, K.J. Methionine regulates copper/hydrogen peroxide oxidation products of Aβ. J. Pept. Sci 2005, 11, 353–360. [Google Scholar]
- Ryu, J.; Joung, H.A.; Kim, M.G.; Park, C.B. Surface plasmon resonance analysis of Alzheimer’s β-amyloid aggregation on a solid surface: from monomers to fully-grown fibrils. Anal. Chem 2008, 80, 2400–2407. [Google Scholar]
- Xia, N.; Liu, L.; Harrington, M.G.; Wang, J.; Zhou, F. Regenerable and simultaneous surface plasmon resonance detection of Aβ(1–40) and Aβ(1–42) peptides in cerebrospinal fluids with signal amplification by streptavidin conjugated to an N-terminus-specific antibody. Anal. Chem 2010, 82, 10151–10157. [Google Scholar]
- Aguilar, M.I.; Small, D.H. Surface plasmon resonance for the analysis of β-amyloid interactions and fibril formation in Alzheimer’s disease research. Neurotox. Res 2005, 7, 17–27. [Google Scholar]
- Krazinski, B.E.; Radecki, J.; Radecka, H. Surface plasmon resonance based biosensors for exploring the influence of alkaloids on aggregation of amyloid-β peptide. Sensors 2011, 11, 4030–4042. [Google Scholar]
- Yao, F.; He, J.; Li, X.; Zou, H.; Yuan, Z. Studies of interaction of copper and zinc ions with Alzheimer’s Aβ(1–16) using surface plasmon resonance spectrometer. Sens. Actuators B 2012, 161, 886–891. [Google Scholar]
- Saber, R.; Piskin, E. Investigation of complexation of immobilized metallothionein with Zn(II) and Cd(II) ions using piezoelectric crystals. Biosens. Bioelectron 2003, 18, 1039–1046. [Google Scholar]
- Stellato, F.; Menestrina, G.; Serra, M.D.; Potrich, C.; Tomazzolli, R.; Meyer-Klaucke, W.; Morante, S. Metal binding in amyloid β-peptides shows intra- and inter-peptide coordination modes. Eur. Biophys. J 2006, 35, 340–351. [Google Scholar]
- Syme, C.D.; Viles, J.H. Solution 1 H NMR investigation of Zn2+ and Cd2+ binding to amyloid-β peptide (Aβ) of Alzheimer’s disease. Biochim. Biophys. Acta 2006, 1764, 246–256. [Google Scholar]
- Miller, Y.; Ma, B.; Nussinov, R. Zinc ions promote Alzheimer Aβ aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. USA 2010, 107, 9490–9495. [Google Scholar]
- Clements, A.; Allsop, D.; Walsh, D.M.; Williams, C.H. Aggregation and metal-binding properties of mutant forms of the amyloid Aβ peptide of Alzheimer’s disease. J. Neurochem 1996, 66, 740–747. [Google Scholar]
- Atwood, C.S.; Scarpa, R.C.; Huang, X.; Moir, R.D.; Jones, W.D.; Fairlie, D.P.; Tanzi, R.E.; Bush, A.I. Characterization of copper interactions with alzheimer amyloid β peptides: Identification of an attomolar-affinity copper binding site on amyloid β1-42. J. Neurochem 2000, 75, 1219–1233. [Google Scholar]
- Damante, C.A.; Osz, K.; Nagy, Z.; Pappalardo, G.; Grasso, G.; Impellizzeri, G.; Rizzarelli, E.; Sovago, I. Metal loading capacity of Aβ N-terminus: A combined potentiometric and spectroscopic study of zinc(II) complexes with Aβ(1–16), its short or mutated peptide fragments and its polyethylene glycol-ylated analogue. Inorg. Chem 2009, 48, 10405–10415. [Google Scholar]
- Karr, J.W.; Akintoye, H.; Kaupp, L.J.; Szalai, V.A. N-Terminal deletions modify the Cu2+ binding site in amyloid-β. Biochemistry 2005, 44, 5478–5487. [Google Scholar]
- Tõugu, V.; Karafin, A.; Palumaa, P. Binding of zinc(II) and copper(II) to the full-length Alzheimer’s amyloid-β peptide. J. Neurochem 2008, 104, 1249–1259. [Google Scholar]
- Faller, P.; Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Trans 2009, 7, 1080–1094. [Google Scholar]
- Findeis, M.A. The role of amyloid β peptide 42 in Alzheimer’s disease. Pharmacol. Ther 2007, 116, 266–286. [Google Scholar]
- Walsh, D.M.; Lomakin, A.; Benedek, G.B.; Condron, M.M.; Teplow, D.B. Amyloid β-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Biol. Chem 1997, 272, 22364–22372. [Google Scholar]
- Hu, W.P.; Chang, G.L.; Chen, S.J.; Kuo, Y.M. Kinetic analysis of β-amyloid peptide aggregation induced by metal ions based on surface plasmon resonance biosensing. J. Neurosci. Meth 2006, 154, 190–197. [Google Scholar]
- Kremer, J.J.; Pallitto, M.M.; Sklansky, D.J.; Murphy, R.M. Correlation of β-amyloid aggregate size and hydrophobicity with decreased bilayer fluidity of model membranes. Biochemistry 2000, 39, 10309–10318. [Google Scholar]




© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yao, F.; Zhang, R.; Tian, H.; Li, X. Studies on the Interactions of Copper and Zinc Ions with β-Amyloid Peptides by a Surface Plasmon Resonance Biosensor. Int. J. Mol. Sci. 2012, 13, 11832-11843. https://doi.org/10.3390/ijms130911832
Yao F, Zhang R, Tian H, Li X. Studies on the Interactions of Copper and Zinc Ions with β-Amyloid Peptides by a Surface Plasmon Resonance Biosensor. International Journal of Molecular Sciences. 2012; 13(9):11832-11843. https://doi.org/10.3390/ijms130911832
Chicago/Turabian StyleYao, Fujun, Ruiping Zhang, He Tian, and Xiangjun Li. 2012. "Studies on the Interactions of Copper and Zinc Ions with β-Amyloid Peptides by a Surface Plasmon Resonance Biosensor" International Journal of Molecular Sciences 13, no. 9: 11832-11843. https://doi.org/10.3390/ijms130911832



