Ethyl Gallate Induces Apoptosis of HL-60 Cells by Promoting the Expression of Caspases-8, -9, -3, Apoptosis-Inducing Factor and Endonuclease G
Abstract
:1. Introduction
2. Results and Discussion
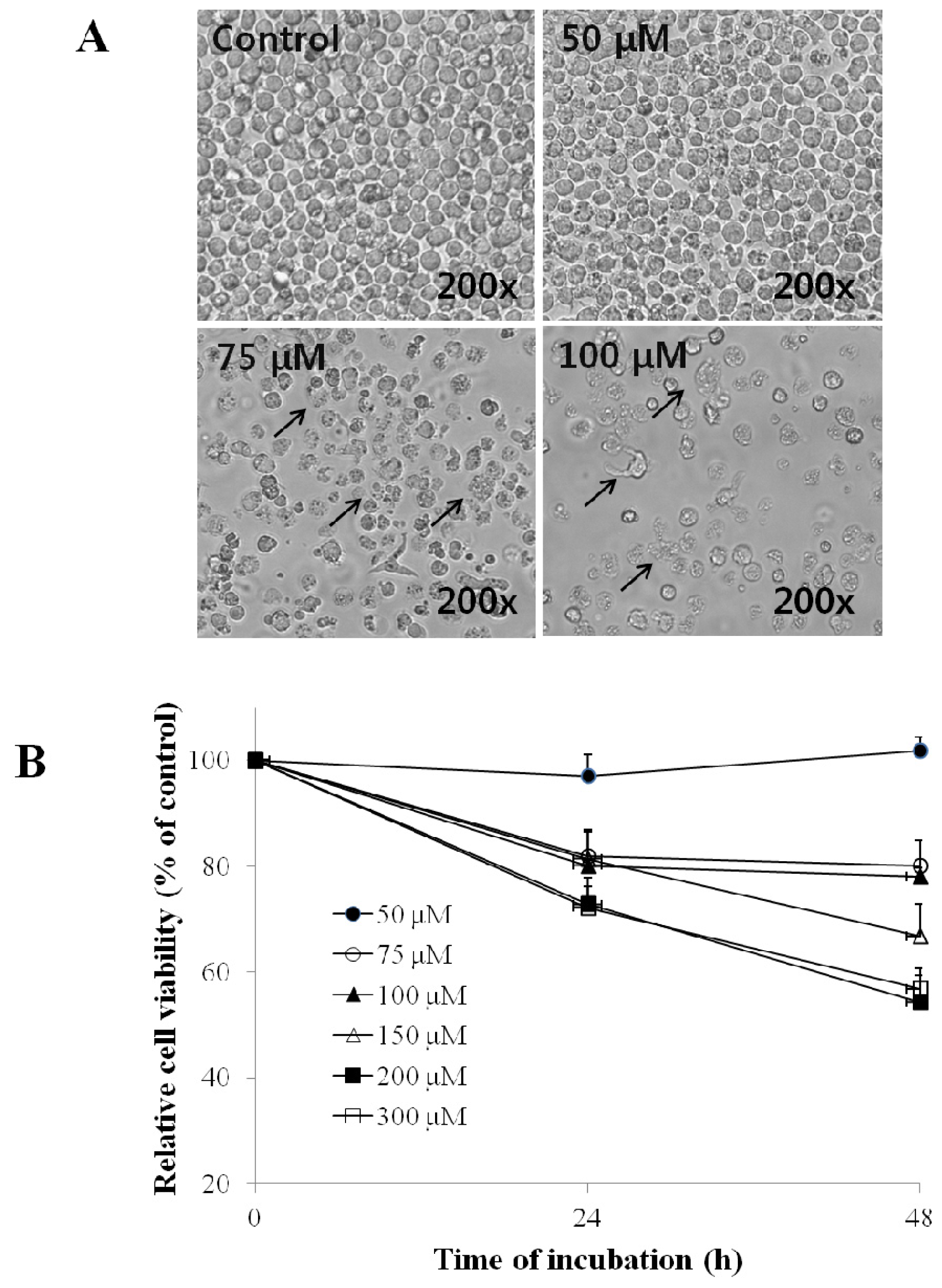
2.1. Effect of EG on the Morphology and Viability of HL-60 Cells
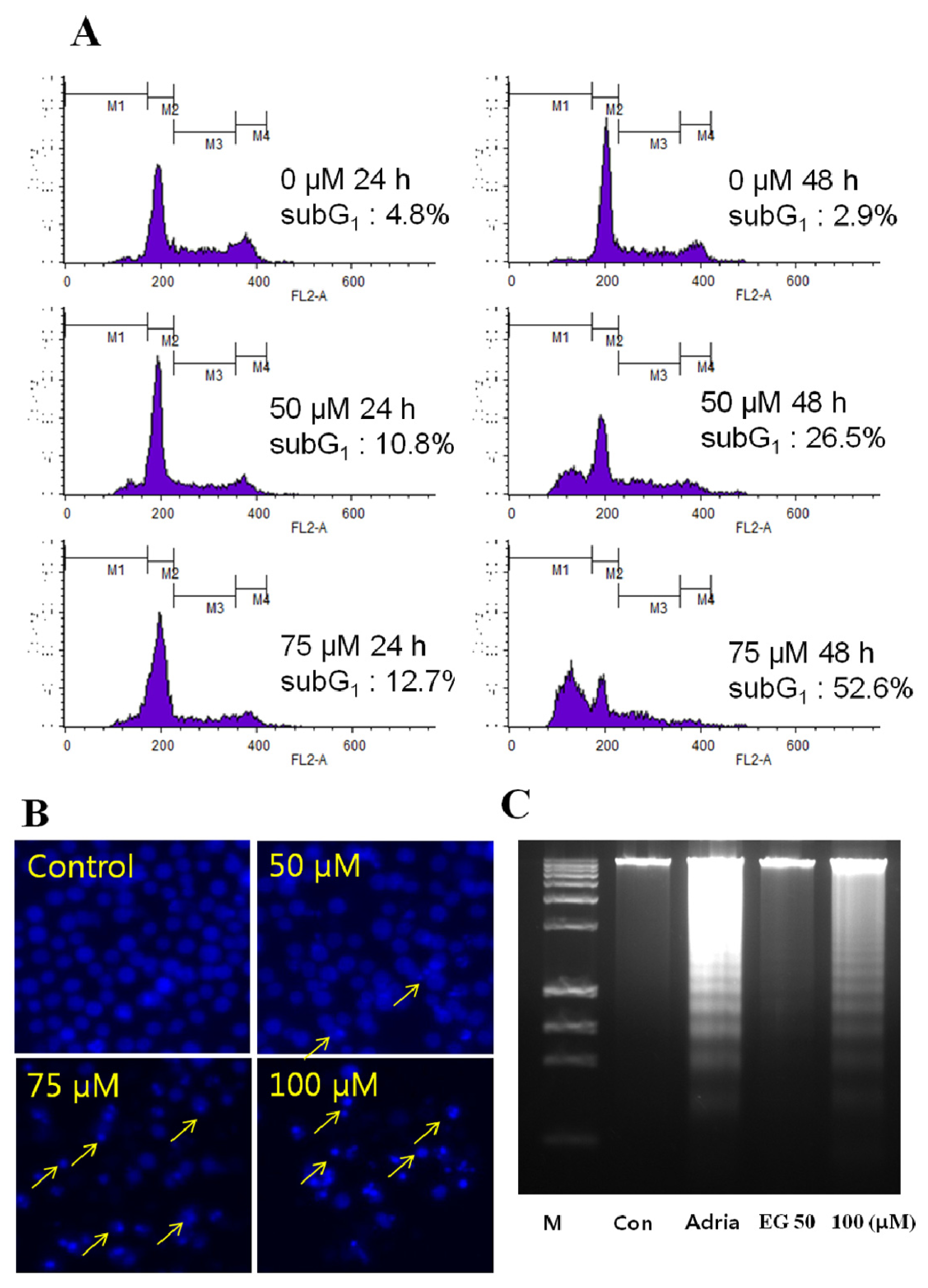
2.2. Analysis of EG-Induced Apoptosis in HL-60 Cells
2.3. Effect of EG on the Expression of Apoptosis-Associated Proteins in HL-60 Cells
3. Materials and Methods
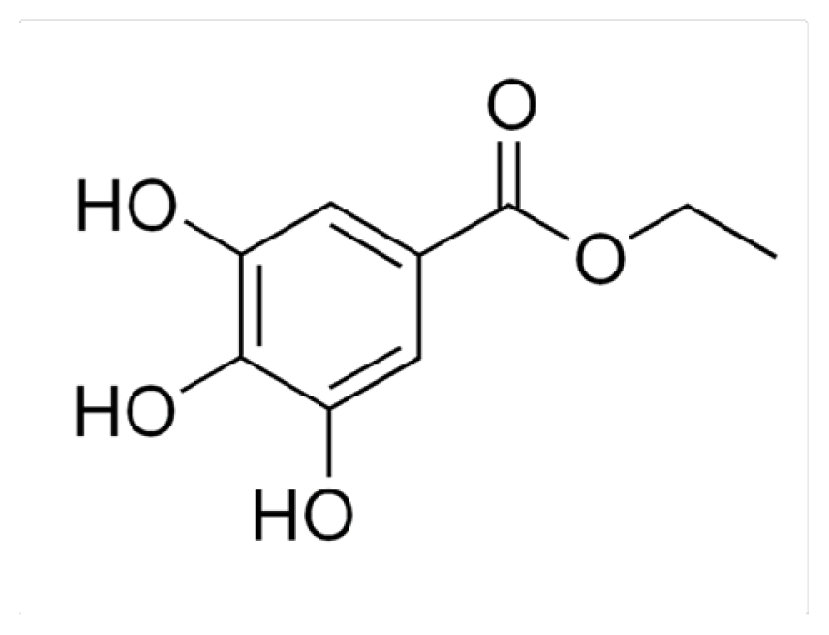
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Cell Viability
3.4. Cell Cycle Analysis by Flow Cytometry and DAPI Staining of Apoptotic Cells
3.5. DNA Fragmentation Analysis
3.6. Western Blotting Analysis
4. Conclusion
Acknowledgements
References
- Rakshit, S.; Mandal, L.; Pal, B.C.; Bagchi, J.; Biswas, N.; Chaudhuri, J.; Chowdhury, A.A.; Manna, A.; Chaudhuri, U.; Konar, A.; et al. Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochem. Pharmacol 2010, 80, 1662–1675. [Google Scholar]
- Hsu, J.L.; Pan, S.L.; Ho, Y.F.; Hwang, T.L.; Kung, F.L.; Guh, J.H. Costunolide induces apoptosis through nuclear calcium2+ overload and DNA damage response in human prostate cancer. J. Urol 2011, 185, 1967–1974. [Google Scholar]
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta Bioenerg 2011, 1807, 735–745. [Google Scholar]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod 2003, 66, 1022–1037. [Google Scholar]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 2010, 29, 405–434. [Google Scholar]
- Kandasamy, K.; Srinivasula, S.M.; Alnemri, E.S.; Thompson, C.B.; Korsmeyer, S.J.; Bryant, J.L.; Srivastava, R.K. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: Differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res 2003, 63, 1712–1721. [Google Scholar]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X.D. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol 1999, 15, 269–290. [Google Scholar]
- Earnshaw, W.C.; Martins, L.M.; Kaufmann, S.H. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu. Rev. Cell Dev. Biol 1999, 68, 383–424. [Google Scholar]
- Shibata, H.; Kondo, K.; Katsuyama, R.; Kawazoe, K.; Sato, Y.; Murakami, K.; Shibata, H.; Kondo, K.; Katsuyama, R.; Kawazoe, K.; et al. Alkyl gallates, intensifiers of beta-lactam susceptibility in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother 2005, 49, 549–555. [Google Scholar]
- Lakshminarasimhan, M.; Steegborn, C. Emerging mitochondrial signaling mechanisms in physiology, aging processes, and as drug targets. Exp. Gerontol 2011, 46, 174–177. [Google Scholar]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria as targets for cancer chemotherapy. Semin. Cancer Biol 2009, 19, 57–66. [Google Scholar]
- Mayevsky, A. Mitochondrial function and energy metabolism in cancer cells: Past overview and future perspectives. Mitochondrion 2009, 9, 165–179. [Google Scholar]
- Chiang, P.C.; Lin, S.C.; Pan, S.L.; Kuo, C.H.; Tsai, I.L.; Kuo, M.T.; Wen, W.C.; Chen, P.; Guh, J.H. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: A crucial role of AMPK and mTOR pathways. Biochem. Pharmacol 2010, 79, 162–171. [Google Scholar]
- Leber, B.; Geng, F.; Kale, J.; Andrews, D.W. Drugs targeting Bcl-2 family members as an emerging strategy in cancer. Expert Rev. Mol. Med 2010, 12, e28. [Google Scholar]
- Rowinsky, E.K.; Onetto, N.; Canetta, R.M.; Arbuck, S.G. Taxol: The first of the taxanes, an important new class of antitumor agents. Semin. Oncol 1992, 19, 646–662. [Google Scholar]
- Weiss, R.B.; Donehower, R.C.; Wiernik, P.H.; Ohnuma, T.; Gralla, R.J.; Trump, D.L.; Baker, J.J.R.; van Echo, D.A; von Hoff, D.D.; Leyland-Jones, B. Hypersensitivity reactions from taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A.; Cremophor, EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar]
- Ahn, Y.J.; Lee, C.O.; Kweon, J.H.; Ahn, J.W.; Park, J.H. Growth-inhibitory effects of Galla Rhois derived tannins on intestinal bacteria. J. Appl. Microbiol 1998, 84, 439–443. [Google Scholar]
- Ata, N.; Oku, T.; Hattori, M.; Fujii, H.; Nakajima, M.; Saiki, I. Inhibition by galloylglucose (GG6-10) of tumor invasion through extracellular matrix and gelatinase-mediated degradation of type IV collagens by metastatic tumor cells. Oncol. Res 1996, 8, 503–511. [Google Scholar]
- Park, E.J.; Zhao, Y.Z.; An, R.B.; Kim, Y.C.; Sohn, D.H. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose from Galla Rhois protects primary rat hepatocytes from necrosis and apoptosis. Planta Med 2008, 74, 1380–1383. [Google Scholar]
- Yoshioka, K.; Kataoka, T.; Hayashi, T.; Hasegawa, M.; Ishi, Y.; Hibasami, H. Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO 205 cell lines. Oncol. Rep 2000, 7, 1221–1223. [Google Scholar]
- Zheng, G.; Xu, L.; Wu, P.; Xie, H.; Jiang, Y.; Chen, F.; Wei, X. Polyphenols from longan seeds and their radical-scavenging activity. Food Chem 2009, 116, 433–436. [Google Scholar]
- Mota, M.L.; Thomas, G.; Barbosa Filho, J.M. Anti-inflammatory actions of tannins isolated from the bark of Anacardium occidentale L. J. Ethnopharmacol 1985, 13, 289–300. [Google Scholar]
- Uchiumi, F.; Maruta, H.; Inoue, J.; Yamamoto, T.; Tanuma, S. Inhibitory effect of tannic acid on human immunodeficiency virus promoter activity induced by 12-O-tetra decanoylphorbol-13-acetate in Jurkat T-cells. Biochem. Biophys. Res. Commun 1996, 220, 411–417. [Google Scholar]
- Feldman, K.S.; Sahasrabudhe, K.; Lawlor, M.D.; Wilson, S.L.; Lang, C.H.; Scheuchenzuber, W.J. In vitro and in vivo inhibition of LPS-stimulated tumor necrosis factor-alpha secretion by the gallotannin beta-d-pentagalloylglucose. Bioorg. Med. Chem. Lett 2011, 11, 1813–1815. [Google Scholar]
- Prakobwong, S.; Gupta, S.C.; Kim, J.H.; Sung, B.; Pinlaor, P.; Hiraku, Y.; Wongkham, S.; Sripa, B.; Pinlaor, S.; Aggarwal, B.B. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis 2011, 32, 1372–1380. [Google Scholar]
- Yeh, R.D.; Chen, J.C.; Yuanlai, T.; Yang, J.S.; Yu, C.S.; Chiang, J.H.; Lu, C.C.; Yang, S.T.; Yu, C.C.; Chang, S.J.; et al. Gallic acid induces G0/G1 phase arrest and apoptosis in human leukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer Res 2011, 31, 2821–2832. [Google Scholar]
- Nunez, G.; Benedict, M.A.; Hu, Y.; Inohara, N. Caspases: The proteases of the apoptotic pathway. Oncogene 1998, 17, 3237–3245. [Google Scholar]
- Brenner, C.; Kroemer, G. Apoptosis. Mitochondria—The death signal integrators. Science 2000, 289, 1150–1151. [Google Scholar]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar]
- Zanna, C.; Ghelli, A.; Porcelli, A.M.; Martinuzzi, A.; Carelli, V.; Rugolo, M. Caspase-independent death of Leber’s hereditary optic neuropathy cybrids is driven by energetic failure and mediated by AIF and Endonuclease G. Apoptosis 2005, 10, 997–1007. [Google Scholar]
- Cregan, S.P.; Fortin, A.; MacLaurin, J.G.; Callaghan, S.M.; Cecconi, F.; Yu, S.W.; Dawson, T.M.; Dawson, V.L.; Park, D.S.; Kroemer, G.; et al. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J. Cell Biol. 2002, 158, 507–517. [Google Scholar]
- Yu, F.S.; Yang, J.S.; Yu, C.S.; Lu, C.C.; Chiang, J.H.; Lin, C.W.; Chung, J.G. Safrole induces apoptosis in human oral cancer HSC-3 cells. J. Dent. Res 2011, 90, 168–174. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, W.-H.; Song, H.-O.; Choi, H.-J.; Bang, H.-I.; Choi, D.-Y.; Park, H. Ethyl Gallate Induces Apoptosis of HL-60 Cells by Promoting the Expression of Caspases-8, -9, -3, Apoptosis-Inducing Factor and Endonuclease G. Int. J. Mol. Sci. 2012, 13, 11912-11922. https://doi.org/10.3390/ijms130911912
Kim W-H, Song H-O, Choi H-J, Bang H-I, Choi D-Y, Park H. Ethyl Gallate Induces Apoptosis of HL-60 Cells by Promoting the Expression of Caspases-8, -9, -3, Apoptosis-Inducing Factor and Endonuclease G. International Journal of Molecular Sciences. 2012; 13(9):11912-11922. https://doi.org/10.3390/ijms130911912
Chicago/Turabian StyleKim, Woong-Hyun, Hyun-Ok Song, Hwa-Jung Choi, Ho-Il Bang, Du-Young Choi, and Hyun Park. 2012. "Ethyl Gallate Induces Apoptosis of HL-60 Cells by Promoting the Expression of Caspases-8, -9, -3, Apoptosis-Inducing Factor and Endonuclease G" International Journal of Molecular Sciences 13, no. 9: 11912-11922. https://doi.org/10.3390/ijms130911912



