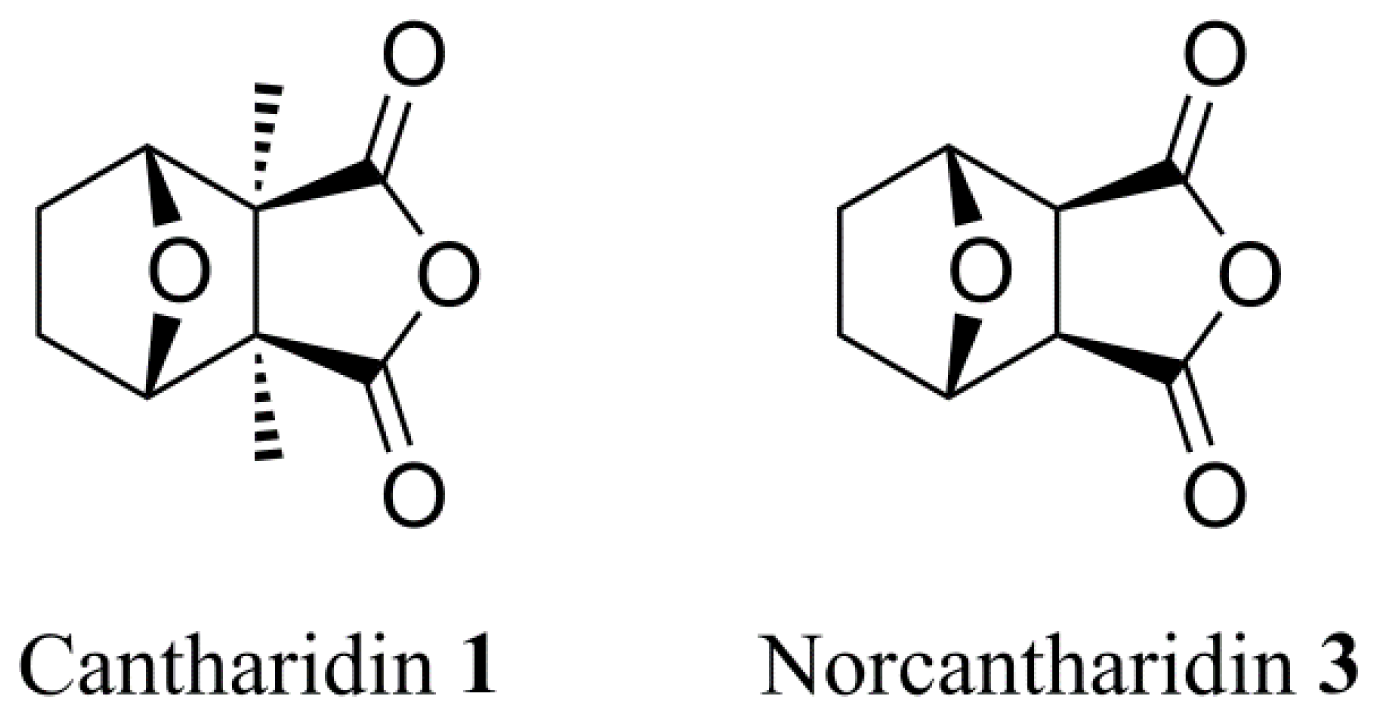
3.2. Synthetic Procedures
3.2.1. General Synthetic Procedure for Compound 2
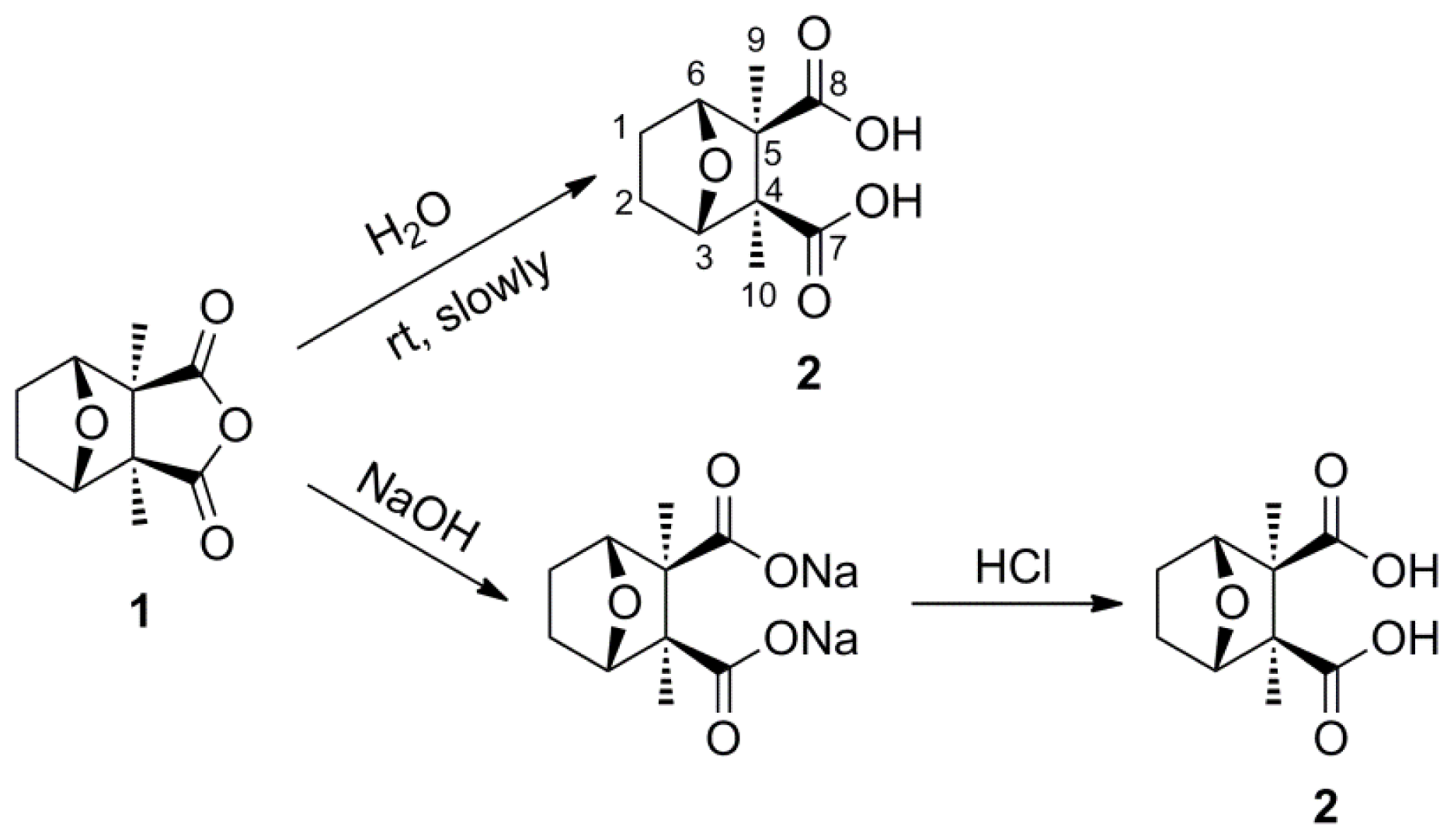
To a solution of cantharidin 1 (390 mg, 2 mmol) in water (10 mL) was dropwise added a solution of sodium hydroxide (180 mg, 4.5 mmol) in water (5 mL). This was stirred at 100 °C for 2 h. After the mixture had cooled to room temperature, a solution of hydrogen chloride (4.5 mmol) in water (9 mL) was added dropwise to the mixture and stirred overnight at room temperature. The white slurry was filtered and the cake washed with dichloromethane. The crude solid was recrystallized from methanol to give 2 as white solid (400 mg, 93%). mp: 232–233 °C. 1H NMR (500 MHz, D2O) δ: 1.03 (s, 6H, H-9, 10), 1.44 (d, J = 8.51 Hz, 2H, H-1, 2, exo), 1.76 (d, J = 7.88 Hz, 2H, H-1, 2, endo), 4.53 (br, s, 2H, H-3, 6). 13C NMR (125 MHz, D2O) δ: 17.26 (C-9, C-10), 23.98 (C-1, C-2), 58.45 (C-4, C-5), 84.29 (C-3, C-6), 183.94 (C-7, C-8). HR-MS (ESI): m/z calcd for C10H13O5 ([M−1]−) 213.0763, found 213.0773.
3.2.2. General Synthetic Procedure for Compound 4
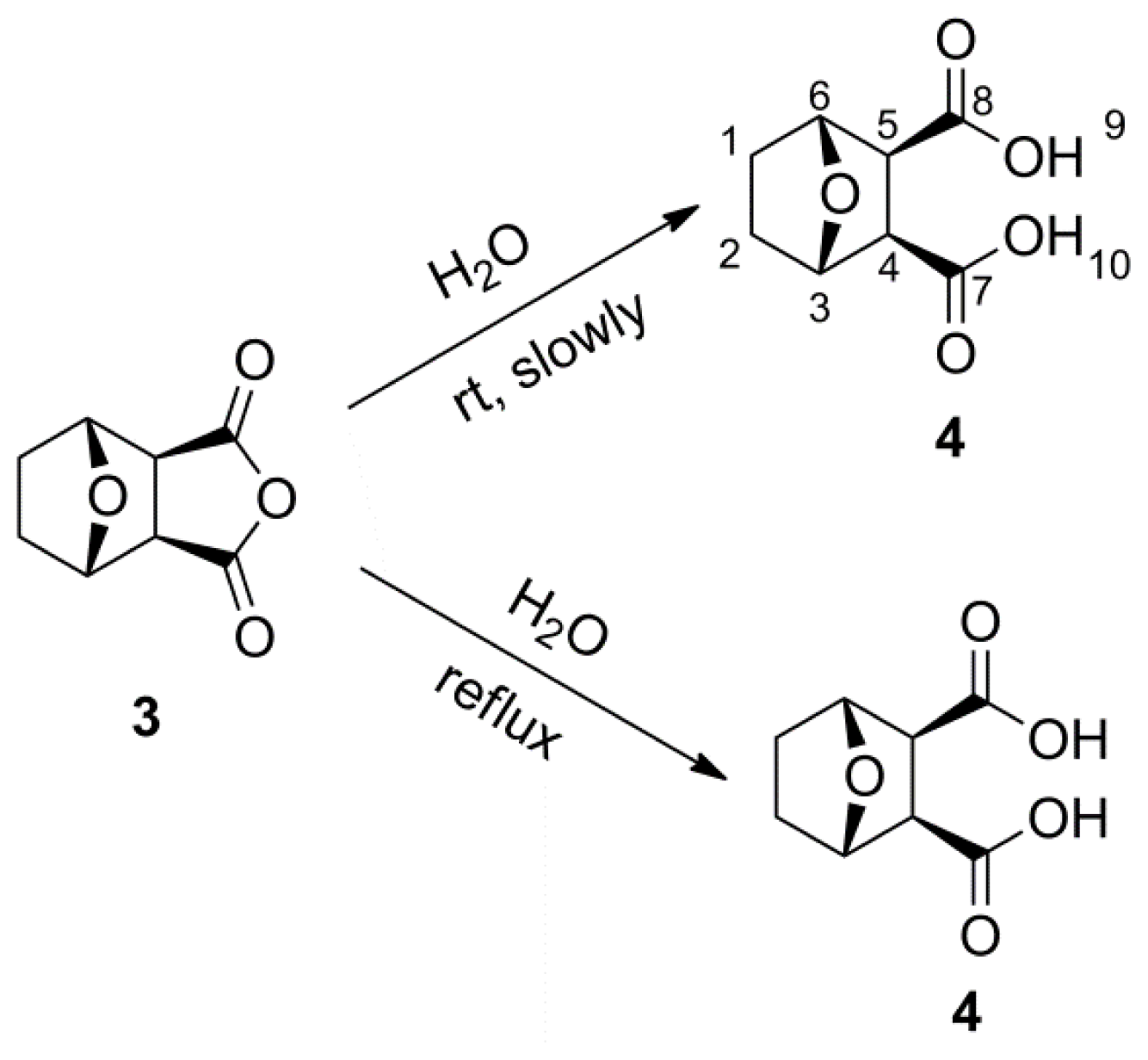
A mixture of norcantharidin 3 (1.0 g, 5.95 mmol) and water (15 mL) was refluxed for 8 h. After the mixture had cooled to room temperature, the reaction was concentrated in vacuo and filtered. The resulting precipitate was recrystallized from methanol to obtain compound 4 as white solid (960 mg, 87%). mp: 137–138 °C. 1H NMR (500 MHz, DMSO-d6) δ: 1.48–1.60 (m, 4H, H-1, 2), 2.92 (s, 2H, H-4, 5), 4.67 (d, J = 2.21 Hz, 2H, H-3, 6), 12.05 (br, s, 2H, H-9, 10). 13C NMR (125 MHz, DMSO-d6) δ: 29.08 (C-1, C-2), 52.03 (C-4, C-5), 78.20 (C-3, C-6), 172.89 (C-7, C-8). HR-MS (ESI): m/z calcd for C8H9O5 ([M−1]−) 185.0450, found 185.0455.
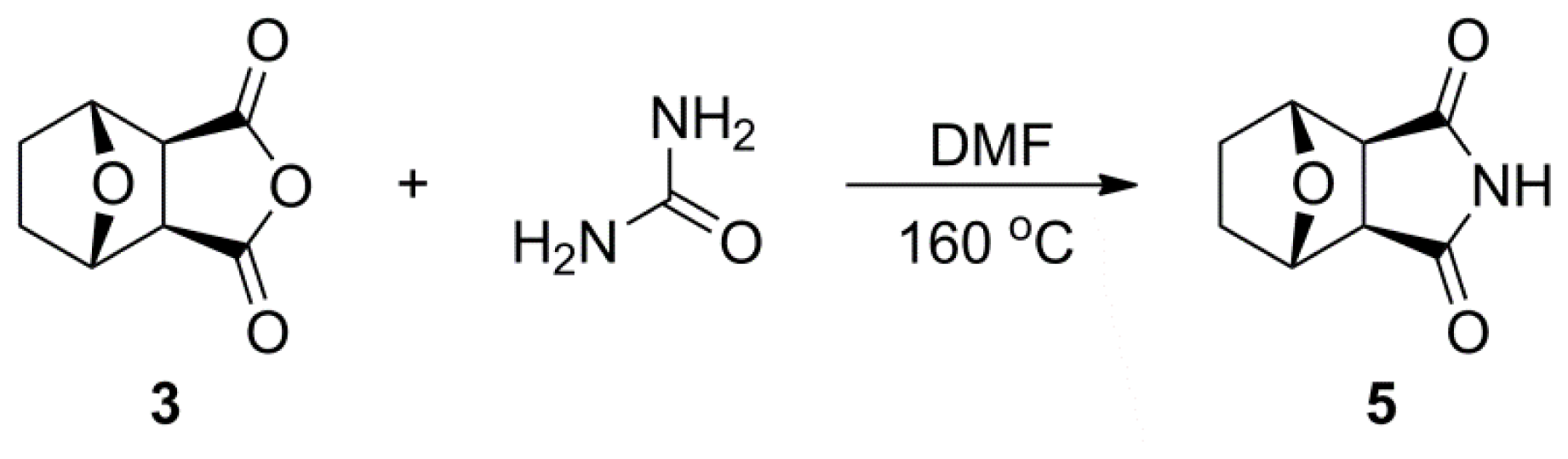
3.2.3. General Synthetic Procedure for Compound 5
A mixture of norcantharidin 3 (1.0 g, 5.95 mmol), carbamide (220 mg, 3.57 mmol) and DMF (1.4 mL) was heated at 130–135 °C. After the mixture had melted completely, it was allowed to react at 160–165 °C. When the reaction was completed according to TLC analysis, the mixture cooled to room temperature. After filtration, the crude solid was recrystallized from EtOAc/Hexane (1:1, v/v) resulting in product 5 as yellow solid (920 mg, 88.5%). mp: 186–188 °C. 1H NMR (500 MHz, CDCl3) δ: 1.60 (d, J = 7.57 Hz, 2H, H-1, 2, exo), 1.87 (d, J = 7.57 Hz, 2H, H-1, 2, endo), 2.93 (br, s, 2H, H-4, 5), 4.92 (br, s, 2H, H-3, 6), 8.33 (br, s, 1H, H-9). 13C NMR (125 MHz, CDCl3) δ: 28.52 (C-1, C-2), 51.36 (C-4, C-5), 79.12 (C-3, C-6), 177.23 (C-7, C-8). HR-MS (ESI): m/z calcd for C8H9NO3Na ([M+Na]+) 190.0480, found 190.0474.
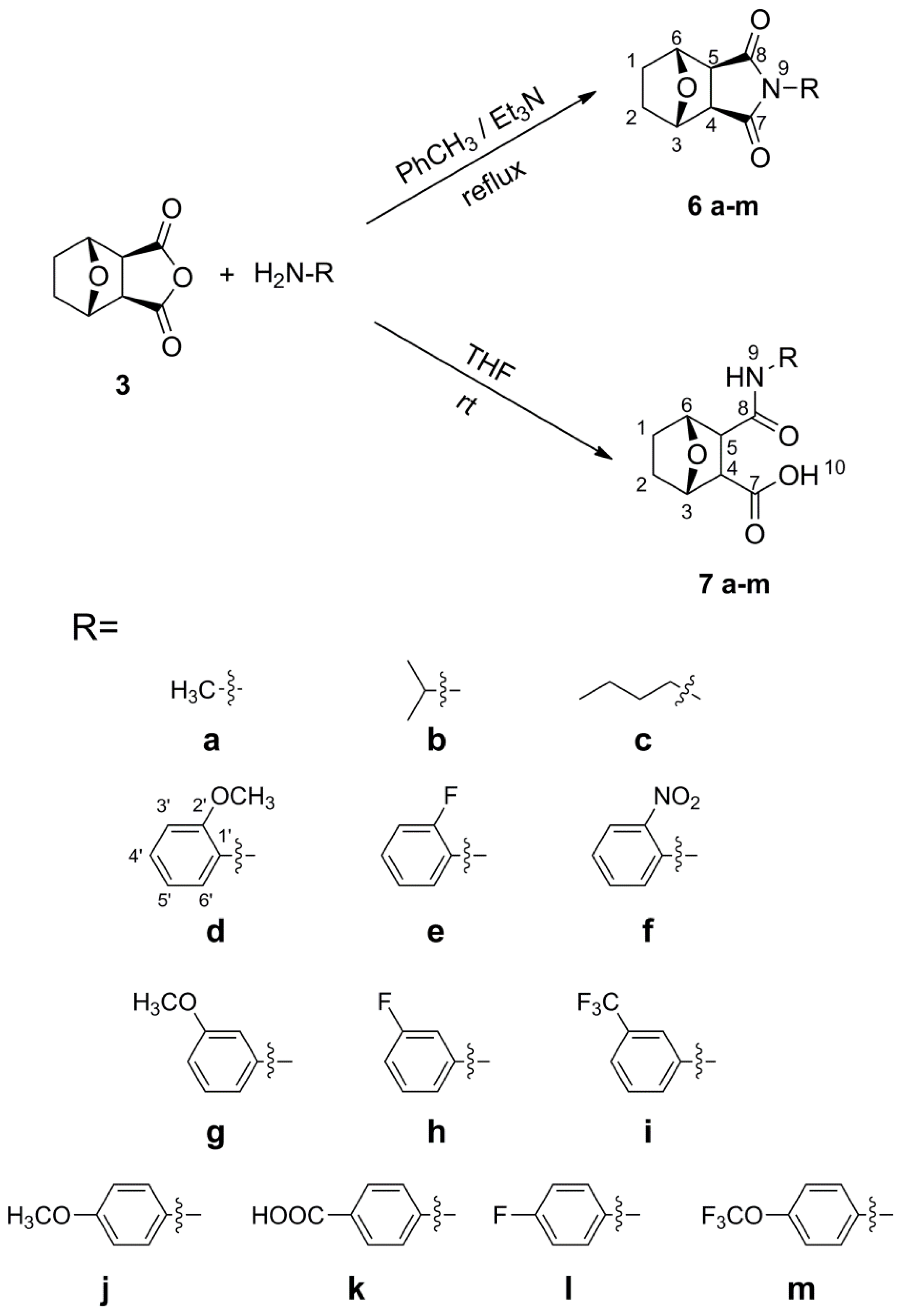
3.2.4. General Synthetic Procedure for the Target Compounds 6 (a–m)
Primary amine (1 equiv, 5.59 mmol) was added to a stirred mixture of norcantharidin 3 (1.0 g, 5.95 mmol), triethylamine (1.5 mL) and toluene (15 mL). The reaction was carried out at reflux temperature, and the progress was monitored by TLC. After completion, the mixture was moved to vacuo. The residue was either recrystallized from EtOAc/Hexane (1:1, v/v) or purified by column chromatography on a silica gel using EtOAc and hexane (EtOAc/Hexane, 2:5, v/v) as the eluent to afford the target compounds 6. The yields, physical properties, 1H NMR, 13C NMR, and HR-MS of the target compounds 6 were as follows:
Data for6-a: white solid; yield, 93%; mp: 132–133 °C; 1H NMR (500 MHz, CDCl3) δ: 1.58–1.64 (m, 2H, H-1, 2, exo), 1.83–1.90 (m, 2H, H-1, 2, endo), 2.89 (s, 2H, H-4, 5), 2.96 (s, 3H, H-1′), 4.88 (br, s, 2H, H-3, 6); 13C NMR (125 MHz, CDCl3) δ: 25.07 (C-1′), 28.59 (C-1, C-2), 50.03 (C-4, C-5), 79.00 (C-3, C-6), 177.26 (C-7, C-8); HR-MS (ESI): m/z calcd for C9H11NO3Na ([M+Na]+) 204.0637, found 204.0633.
Data for6-b: white solid; yield, 71%; mp: 141–142 °C; 1H NMR (500 MHz, CDCl3) δ: 1.36 (d, J = 6.94 Hz, 6H, H-2′, 3′), 1.56–1.62 (m, 2H, H-1, 2, exo), 1.80–1.88 (m, 2H, H-1, 2, endo), 2.79 (s, 2H, H-4, 5), 4.32 (dt, J = 13.87, 6.94 Hz, 1H, H-1′), 4.82–4.89 (m, 2H, H-3, 6); 13C NMR (125 MHz, CDCl3) δ: 19.16 (s, 2C, C-2′, C-3′), 28.63 (s, 2C, C-1, C-2), 43.99 (s, 1C, C-1′), 49.58 (s, 2C, C-4, C-5), 79.18 (s, 2C, C-3, C-6), 177.27 (s, 2C, C-7, C-8); HR-MS (ESI): m/z calcd for C11H15NO3Na ([M+Na]+) 232.0950, found 232.0953.
Data for6-c: white solid; yield, 89%; mp: 80–81 °C; 1H NMR (500 MHz, CDCl3) δ: 0.91 (t, J = 7.25 Hz, 3H, H-4′), 1.29 (sxt, J = 7.31 Hz, 2H, H-3′), 1.52 (quin, J = 7.41 Hz, 2H, H-2′), 1.60 (d, J = 7.25 Hz, 2H, H-1, 2, exo), 1.86 (d, J = 7.88 Hz, 2H, H-1, 2, endo), 2.86 (s, 2H, H-4, 5), 3.46 (t, J = 7.25 Hz, 2H, H-1′), 4.87 (br, s, 2H, H-3, 6); 13C NMR (125 MHz, CDCl3) δ: 13.62 (C-4′), 19.94 (C-3′), 28.61 (C-1, C-2), 29.65 (C-2′), 38.88 (C-1′), 49.87 (C-4, C-5), 79.07 (C-3, C-6), 177.28 (C-7, C-8); HR-MS (ESI): m/z calcd for C12H17NO3Na ([M+Na]+) 246.1106, found 246.1101.
Data for6-d: white solid; yield, 71%; mp: 149–150 °C; 1H NMR (500 MHz, CDCl3) δ: 1.65 (d, J = 7.25 Hz, 2H, H-1, 2, exo), 1.90 (d, J = 7.88 Hz, 2H, H-1, 2, endo), 3.00–3.10 (m, 2H, H-4, 5), 3.76–3.82 (m, 3H, H-7′), 4.99 (d, J = 2.84 Hz, 2H, H-3, 6), 6.97–7.05 (m, 2H, H-3′, 4′), 7.06–7.17 (m, 1H, H-5′), 7.39 (t, J = 7.72 Hz, 1H, H-6′); 13C NMR (125 MHz, CDCl3) δ: 28.66 (C-1, C-2), 50.41 (C-4, C-5), 55.81 (C-7′), 79.43 (C-3, C-6), 112.09 (C-3′), 112.57 (C-6′), 120.98 (C-1′), 129.32 (C-5′), 130.84 (C-4′), 154.57 (C-2′), 176.28 (C-7, C-8); HR-MS (ESI): m/z calcd for C15H15NO4Na ([M+Na]+) 296.0899, found 296.1001.
Data for6-e: white solid; yield, 71%; mp: 139–140 °C; 1H NMR (500 MHz, CDCl3) δ: 1.62–1.70 (m, 2H, H-1, 2, exo), 1.87–1.95 (m, 2H, H-1, 2, endo), 3.09 (br, s, 2H, H-4, 5), 5.00 (br, s, 2H, H-3, 6), 7.18–7.22 (m, 1H, H-4′), 7.23 (br, s, 2H, H-3′, 5′), 7.37–7.45 (m, 1H, H-6′); 13C NMR (125 MHz, CDCl3) δ: 28.64 (s, 2C, C-1, C-2), 50.39 (s, 2C, C-4, C-5), 79.51 (s, 2C, C-3, C-6), 119.65 (s, 1C, C-3′), 124.67 (br, s, 1C, C-5′), 128.24 (s, 1C, C-1′), 129.42 (br. s, 1C, C-4′), 131.01 (s, 1C, C-6′), 156.29 (s, 1C, C-2′), 175.54 (s, 2C, C-7, C-8); HR-MS (ESI): m/z calcd for C14H12FNO3Na ([M+Na]+) 284.0699, found 284.0691.
Data for6-f: white solid; yield, 52%; mp: 179–181 °C; 1H NMR (500 MHz, CDCl3) δ: 1.68 (d, J = 7.25 Hz, 2H, H-1, 2, exo), 1.92 (d, J = 7.88 Hz, 2H, H-1, 2, endo), 3.11–3.21 (m, 2H, H-4, 5), 4.96–5.06 (m, 2H, H-3, 6), 7.41 (d, J = 7.57 Hz, 1H, H-4′), 7.61 (t, J = 7.57 Hz, 1H, H-5′), 7.70–7.80 (m, 1H, H-6′), 8.18 (d, J = 8.20 Hz, 1H, H-3′); 13C NMR (125 MHz, CDCl3) δ: 28.61 (C-1, C-2), 50.59 (C-4, C-5), 79.49 (C-3, C-6), 125.81 (C-6′), 130.19 (C-4′), 130.66 (C-3′), 134.39 (C-5′), 175.47 (C-7, C-8); HR-MS (ESI): m/z calcd for C14H12N2O5Na ([M+Na]+) 311.0644, found 311.0623.
Data for6-g: white solid; yield, 79%; mp: 147–148 °C; 1H NMR (500 MHz, CDCl3) δ: 1.62–1.69 (m, 2H, H-1, 2, exo), 1.87–1.95 (m, 2H, H-1, 2, endo), 3.03 (s, 2H, H-4, 5), 3.81 (s, 3H, H-7′), 5.00 (br, s, 2H, H-3, 6), 6.78 (s, 1H, H-4′), 6.84 (d, J = 7.88 Hz, 1H, H-2′), 6.94 (dd, J = 8.35, 1.73 Hz, 1H, H-6′), 7.36 (t, J = 8.04 Hz, 1H, H-5′); 13C NMR (125 MHz, CDCl3) δ: 28.68 (C-1, C-2), 50.08 (C-4, C-5), 55.48 (C-7′), 79.56 (C-3, C-6), 112.24 (C-2′), 114.91 (C-4′), 118.81 (C-6′), 129.89 (C-5′), 132.85 (C-1′), 160.14 (C-3′), 176.30 (C-7, C-8); HR-MS (ESI): m/z calcd for C15H15NO4Na ([M+Na]+) 296.0899, found 296.1015.
Data for6-h: white solid; yield, 85%; mp: 139–140 °C; 1H NMR (500 MHz, CDCl3) δ: 1.64–1.70 (m, 2H, H-1, 2, exo), 1.87–1.94 (m, 2H, H-1, 2, endo), 3.03 (s, 2H, H-4, 5), 4.99 (d, J = 2.21 Hz, 2H, H-3, 6), 7.11–7.18 (m, 2H, H-4′, 6′), 7.22–7.29 (m, 2H, H-2′, 5′); 13C NMR (125 MHz, CDCl3) δ: 28.66 (s, 2C, C-1, C-2), 50.04 (s, 2C, C-4, C-5), 79.57 (s, 2C, C-3, C-6), 116.09 (s, 1C, C-2′), 116.28 (s, 1C, C-4′), 127.75 (s, 1C, C-6′), 128.35 (s, 1C, C-5′), 161.31 (s, 1C, C-1′), 163.29 (s, 1C, C-3′), 176.27 (s, 2C, C-7, C-8); HR-MS (ESI): m/z calcd for C14H12FNO3Na ([M+Na]+) 284.0699, found 284.0692.
Data for6-i: white solid; yield, 92%; mp: 197–198 °C; 1H NMR (500 MHz, CDCl3) δ: 1.65–1.71 (m, 2H, H-1, 2, exo), 1.89–1.96 (m, 2H, H-1, 2, endo), 3.06 (s, 2H, H-4, 5), 5.01 (d, J = 2.21 Hz, 2H, H-3, 6), 7.50 (d, J = 7.88 Hz, 1H, H-6′), 7.56–7.62 (m, 2H, H-4′, 5′), 7.63–7.68 (m, 1H, H-2′); 13C NMR (125 MHz, CDCl3) δ: 28.65 (s, 2C, C-1, C-2), 50.09 (s, 2C, C-4, C-5), 79.64 (s, 2C, C-3, C-6), 123.56 (s, 1C, C-4′), 124.59 (s, 1C, C-7′), 125.44 (s, 1C, C-2′), 129.70 (s, 1C, C-5′), 131.56 (s, 1C, C-3′), 131.82 (s, 1C, C-6′), 132.38 (s, 1C, C-1′), 175.88 (s, 2C, C-7, C-8); HR-MS (ESI): m/z calcd for C15H12F3NO3Na ([M+Na]+) 334.0667, found 334.0665.
Data for6-j: white solid; yield, 85%; mp: 189–190 °C; 1H NMR (500 MHz, CDCl3) δ: 1.66 (d, J = 7.25 Hz, 2H, H-1, 2, exo), 1.90 (d, J = 8.20 Hz, 2H, H-1, 2, endo), 3.02 (s, 2H, H-4, 5), 3.82 (s, 3H, H-7′), 4.99 (br, s, 2H, 2H, H-3, 6), 6.97 (d, J = 8.83 Hz, 2H, H-3′, 5′), 7.17 (d, J = 8.83 Hz, 2H, H-2′, 6′); 13C NMR (125 MHz, CDCl3) δ: 28.69 (C-1, C-2), 40.48 (C-7′), 49.99 (C-4, C-5), 79.47 (C-3, C-6), 112.44 (C-3′, C-5′), 120.25 (C-1′), 127.17 (C-2′, C-6′), 150.57 (C-4′), 176.98 (C-7, C-8); HR-MS (ESI): m/z calcd for C15H15NO4Na ([M+Na]+) 296.0899, found 296.1011.
Data for6-k: white solid; yield, %; mp: 270–271 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.71 (s, 4H, H-1, 2), 2.94 (q, J = 7.25 Hz, 2H, H-4, 5), 4.83 (s, 2H, H-3, 6), 7.31 (d, J = 8.20 Hz, 2H, H-2′, 6′), 8.03 (d, J = 8.51 Hz, 2H, H-3′, 5′); 13C NMR (125 MHz, DMSO-d6) δ: 28.48 (s, 2C, C-1, C-2), 50.31 (s, 2C, C-4, C-5), 79.37 (s, 2C, C-3, C-6), 126.87 (s, 2C, C-2′, C-6′), 130.27 (s, 2C, C-3′, C-5′), 133.71 (s, 1C, C-4′), 135.51 (s, 1C, C-1′), 167.83 (s, 1C, C-7′), 177.07 (s, 2C, C-7, C-8); HR-MS (ESI): m/z calcd for C30H26N2O102Na ([2M+Na]+) 597.1531, found 597.1535.
Data for6-l: white solid; yield, 71%; mp: 140–141 °C; 1H NMR (500 MHz, CDCl3) δ: 1.63–1.69 (m, 2H, H-1, 2, exo), 1.88–1.94 (m, 2H, H-1, 2, endo), 3.03 (s, 2H, H-4, 5), 4.99 (d, J = 2.21 Hz, 2H, H-3, 6), 7.10–7.19 (m, 2H, H-3′, 5′), 7.22–7.29 (m, 2H, H-2′, 6′); 13C NMR (125 MHz, CDCl3) δ: 28.66 (s, 2C, C-1, C-2), 50.04 (s, 2C, C-4, C-5), 79.57 (s, 2C, C-3, C-6), 116.09 (s, 2C, C-3′, C-5′), 127.75 (s, 1C, C-1′), 128.35 (s, 2C, C-2′, C-6′), 163.29 (s, 1C, C-4′), 176.27 (s, 2C, C-7, C-8); HR-MS (ESI): m/z calcd for C14H12FNO3Na ([M+Na]+) 284.0699, found 284.0675.
Data for6-m: white solid; yield, 85%; mp: 177–178 °C; 1H NMR (500 MHz, CDCl3) δ: 1.67 (d, J = 7.57 Hz, 2H, H-1, 2, exo), 1.92 (d, J = 7.88 Hz, 2H, H-1, 2, endo), 3.05 (s, 2H, H-4, 5), 5.00 (br, s, 2H, H-3, 6), 7.28–7.33 (m, 2H, H-3′, 5′), 7.33–7.38 (m, 2H, H-2′, 6′); 13C NMR (125 MHz, CDCl3) δ: 28.66 (s, 2C, C-1, C-2) 50.05 (s, 2C, C-4, C-5) 79.62 (s, 2C, C-3. C-6) 119.36 (s, 1C, C-1′) 121.60 (s, 2C, C-3′, C-5′) 127.98 (s, 2C, C-2′, C-6′) 130.22 (s, 1C, C-4′) 148.89 (s, 1C, C-7′) 176.07 (s, 2C, C-7, C-8); HR-MS (ESI): m/z calcd for C15H12F3NO4Na ([M+Na]+) 350.0616, found 350.0615.
3.2.5. General Synthetic Procedure for the Target Compounds 7 (a–m)
A mixture of norcantharidin 3 (1.0 g, 5.95 mmol), primary amine regent (1 equiv, 5.95 mmol) and THF (10 mL) was stirred for several hours at room temperature, and the progress of reaction was monitored by TLC. After norcantharidin disappeared, the reaction was concentrated under reduced pressure and diluted with acetone (100 mL). The resulting precipitate was either recrystallized from methanol or purified by column chromatography (MeOH/CH2Cl2, 1:4, v/v) to afford the desired products 7. The yields, physical properties, 1H NMR, 13C NMR, and HR-MS of the target compounds 7 were as follows:
Data for7-a: white solid; yield, 84%; mp: 144–146 °C; 1H NMR (500 MHz, D2O) δ: 1.45–1.70 (m, 4H, H-1, 2), 2.57 (s, 3H, H-1′), 2.88–2.95 (m, 2H, H-4, 5), 4.60 (d, J = 4.73 Hz, 1H, H-6), 4.79 (d, J = 4.41 Hz, 1H, H-3); 13C NMR (125 MHz, D2O) δ: 25.87 (C-1′), 28.20 (C-1, C-2), 53.84 (C-4), 54.70 (C-5), 78.68 (C-3), 79.39 (C-6), 175.58 (C-8), 177.81 (C-7); HR-MS (ESI): m/z calcd for C9H12NO4 ([M−1]−) 198.0766, found 198.0753.
Data for7-b: white solid; yield 74%; mp: 136–137 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.02 (dd, J = 6.31, 2.52 Hz, 6H, H-2′, 3′), 1.41–1.62 (m, 4H, H-1. 2), 2.75–2.85 (m, 2H, H-4, 5), 3.76 (dq, J = 13.52, 6.74 Hz, 1H, H-1′), 4.47 (d, J = 4.73 Hz, 1H, H-6), 4.72 (d, J = 4.10 Hz, 1H, H-3), 7.16 (d, J = 7.88 Hz, 1H, H-9); 13C NMR (125 MHz, DMSO-d6) δ: 22.67 (s, 1C, C-2′), 22.88 (s, 1C, C-3′), 28.93 (s, 1C, C-1), 29.31 (s, 1C, C-2), 43.13 (s, 1C, C-1′), 52.60 (s, 1C, C-3), 53.60 (s, 1C, C-4), 77.41 (s, 1C, C-3), 79.08 (s, 1C, C-6), 170.20 (s, 1C, C-8), 173.07 (s, 1C, C-7); HR-MS (ESI): m/z calcd for C11H18NO4 ([M+1]+) 228.1236, found 228.1233.
Data for7-c: white solid; yield, 83.5%; mp: 139–140 °C; 1H NMR (500 MHz, DMSO-d6) δ: 0.88 (t, J = 7.25 Hz, 3H, H-4′), 1.23–1.32 (m, 2H, H-3′), 1.32–1.40 (m, 2H, H-2′), 1.42–1.63 (m, 4H, H-1, 2), 2.81–2.87 (m, 2H, H-1′), 2.94–3.05 (m, 2H, H-4, 5), 4.48 (d, J = 4.41 Hz, 1H, H-6), 4.74 (d, J = 4.10 Hz, 1H, H-3), 7.33 (t, J = 5.20 Hz, 1H, H-9), 11.89 (br, s, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 14.17 (C-4′), 20.06 (C-3′), 28.88 (C-2), 29.34 (C-1), 31.06 (C-2′), 38.65 (C-1′), 51.89 (C-4), 53.56 (C-5), 77.22 (C-3), 79.28 (C-6), 170.76 (C-8), 172.82 (C-7); HR-MS (ESI): m/z calcd for C12H19NO4Na ([M+Na]+) 264.1212, found 264.1213.
Data for7-d: white solid; yield, 66%; Mp: 151–152 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.52–1.72 (m, 4H, H-1, 2), 3.05–3.17 (m, 2H, H-4, 5), 3.83 (s, 3H, H-7′), 4.71 (d, J = 5.04 Hz, 1H, H-6′), 4.89 (d, J = 3.47 Hz, 1H, H-3′), 6.86–6.93 (m, 1H, H-5′), 7.02 (d, J = 4.10 Hz, 2H, H-3′, 4′), 8.11 (d, J = 7.88 Hz, 1H, H-6′), 8.87 (s, 1H, H-9), 12.20 (br, s, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.96 (C-1, C-2), 52.44 (C-7′), 55.32 (C-4), 56.45 (C-5), 77.52 (C-3), 79.54 (C-6), 111.44 (C-3′), 120.32 (C-6′), 120.79 (C-5′), 123.84 (C-3′), 128.31 (C-4′), 148.84 (C-2′), 169.95 (C-8), 172.61 (C-7); HR-MS (ESI): m/z calcd for C15H17NO5Na ([M+Na]+) 314.1004, found 314.1008.
Data for7-e: white solid; yield, 85%; mp: 114–115 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.53–1.68 (m, 4H, H-1, 2), 3.04 (d, J = 9.46 Hz, 1H, H-4), 3.21 (d, J = 9.77 Hz, 1H, H-5), 4.61–4.77 (m, 1H, H-6), 4.78–4.93 (m, 1H, H-3), 7.06–7.19 (m, 2H, H-3′, 5′), 7.20–7.28 (m, 1H, H-4′), 8.02 (t, J = 7.72 Hz, 1H, H-6′), 9.28 (s, 1H, H-9), 12.13 (br, s, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.92 (s, 1C, C-1), 29.11 (s, 1C, C-2), 52.28 (s, 1C, C-4), 54.13 (s, 1C, C-5), 77.54 (s, 1C, C-3), 79.36 (s, 1C, C-6), 115.53 (s, 1C, C-3′), 123.24 (s, 1C, C-1′), 124.83 (s, 1C, 6′), 126.95 (s, 1C, 4′), 152.24 (s, 1C, C-5′), 154.17 (s, 1C, C-2′), 170.22 (s, 1C, C-8), 172.71 (s, 1C, C-7); HR-MS (ESI): m/z calcd for C14H14FNO4Na ([M+Na]+) 302.0805, found 302.0807.
Data for7-f: yellow solid; yield, 47%; mp: 160–161 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.46–1.72 (m, 4H, H-1, 2), 2.98–3.12 (m, 2H, H-4, 5), 4.63–4.85 (m, 2H, H-3, 6), 7.60 (t, J = 8.20 Hz, 1H, H-4′), 7.82 (d, J = 7.88 Hz, 1H, H-5′), 7.90 (d, J = 7.88 Hz, 1H, H-6′), 8.65 (br, s, 1H, H-3′), 10.28 (br, s, 1H, H-9), 12.02–12.09 (m, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.91 (C-2), 29.44 (C-1), 52.24 (C-4), 53.82 (C-5), 77.49 (C-3), 79.00 (C-6), 113.68 (C-6′), 117.96 (C-4′), 125.55 (C-3′), 130.53 (C-5′), 140.91 (C-1′), 148.42 (C-2′), 170.61 (C-8), 172.63 (C-7); HR-MS (ESI): m/z calcd for C14H14N2O6Na 329.0750, found 329.0754.
Data for7-g: white solid; yield, 73%; mp: 165–166 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.47–1.70 (m, 4H, H-1, 2), 2.95 (d, J = 9.46 Hz, 1H, H-4), 3.07 (d, J = 9.77 Hz, 1H, H-5), 4.64 (d, J = 4.41 Hz, 1H, H-6), 4.80 (d, J = 3.78 Hz, 1H, H-3), 6.61 (dd, J = 8.20, 1.89 Hz, 1H, H-4′), 7.05 (d, J = 8.20 Hz, 1H, H-6′), 7.15–7.22 (m, 1 H, H-5′), 7.29 (s, 1H, H-2′), 9.68 (s, 1H, H-9), 11.99 (br, s, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.91 (C-2), 29.46 (C-1), 52.05 (C-7′), 54.03 (C-4), 55.42 (C-5), 77.34 (C-3), 79.23 (C-6), 105.36 (C-6′), 108.97 (C-2′), 111.89 (C-4′), 129.81 (C-5′), 140.95 (C-1′), 159.95 (C-3′), 169.83 (C-8), 172.72 (C-7); HR-MS (ESI): m/z calcd for C15H17NO5Na ([M+Na]+) 314.1004, found 314.0983.
Data for7-h: white solid; yield, 86%; mp: 163–164 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.41–1.71 (m, 4H, H-1, 2), 2.97 (d, J = 9.46 Hz, 1H, H-4), 3.07 (d, J = 9.46 Hz, 1H, H-5), 4.66 (d, J = 4.41 Hz, 1H, H-6), 4.80 (d, J = 3.78 Hz, 1H, H-3), 6.85 (td, J = 8.43, 2.36 Hz, 1H, H-4′), 7.22 (d, J = 8.20 Hz, 1H, H-6′), 7.29–7.36 (m, 1H, H-5′), 7.57 (d, J = 11.66 Hz, 1H, H-2′), 9.95 (s, 1H, H-9) 12.01 (s, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.89 (s, 1C, C-1), 29.44 (s, 1C, C-2), 52.17 (s, 1C, C-4), 53.89 (s, 1C, C-5), 77.38 (s, 1C, C-3), 79.07 (s, 1C, C-6), 106.28 (s, 1C, C-4′), 109.72 (s, 1C, C-2′), 115.31 (s, 1C, C-6′), 130.62 (s, 1C, C-5′), 141.46 (s, 1C, C-1′), 161.65 (s, 1C, C-3′), 170.19 (s, 1C, C-8), 172.64 (s, 1C, C-7); HR-MS (ESI): m/z calcd for C14H14FNO4Na ([M+Na]+) 302.0805, found 302.0811.
Data for7-i: white solid; yield, 49%; mp: 174–176 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.43–1.76 (m, 4H, H-1, 2), 2.99 (d, J = 9.46 Hz, 1H, H-4), 3.09 (d, J = 9.46 Hz, 1H, H-5), 4.69 (d, J = 2.84 Hz, 1H, H-6), 4.80 (br, s, 1H, H-3), 7.38 (d, J = 7.57 Hz, 1H, H-5′), 7.54 (t, J = 7.88 Hz, 1H, H-4′), 7.68 (d, J = 7.88 Hz, 1H, H-6′), 8.12 (br, s, 1H, H-2′), 10.11 (s, 1H, H-9), 12.04 (s, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.90 (s, 1C, C-1), 29.43 (s, 1C, C-2), 52.19 (s, 1C, C-4), 53.85 (s, 1C, C-5), 77.43 (s, 1C, C-3), 79.04 (s, 1C, C-6), 115.67 (s, 1C, C-4′), 119.70 (s, 1C, C-7′), 123.08 (s, 1C, C-6′), 123.56 (s, 1C, C-2′), 125.72 (s, 1C, C-5′), 130.29 (s, 1C, C-3′), 140.51 (s, 1C, C-1′), 170.43 (s, 1C, C-8), 172.67 (s, 1C, C-7); HR-MS (ESI): m/z calcd for C15H14F3NO4Na ([M+Na]+) 352.0773, found 352.0775.
Data for7-j: white solid; yield, 71%; mp: 167–168 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.46–1.69 (m, 4H, H-1, 2), 2.94 (d, J = 9.77 Hz, 1H, H-4), 3.03 (d, J = 9.46 Hz, 1H, H-5), 4.63 (d, J = 4.10 Hz, 1H, 1H, H-6), 4.79 (d, J = 3.78 Hz, 1H, 1H, H-3), 6.87 (d, J = 8.83 Hz, 2H, H-3′, 5′), 7.44 (d, J = 8.83 Hz, 2 H, H-2′, 6′), 9.51 (s, 1H, H-9), 11.93 (br, s, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.89 (C-2), 29.47 (C-1), 52.07 (C-7′), 53.87 (C-4), 55.64 (C-5), 77.29 (C-3), 79.20 (C-6), 114.20 (C-3′, C-5′), 121.24 (C-2′, C-6′), 132.91 (C-1′), 155.54 (C-4′), 169.33 (C-8), 172.76 (C-7); HR-MS (ESI): m/z calcd for C15H17NO5Na ([M+Na]+) 314.1004, found 314.1025.
Data for7-k: white solid; yield, 44%; mp: 269–270 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.52–1.64 (m, 4H, H-1, 2), 2.98 (d, J = 9.46 Hz, 1H, H-4), 3.11 (d, J = 9.77 Hz, 1H, H-5), 4.68 (d, J = 4.41 Hz, 1H, H-6), 4.80 (d, J = 3.78 Hz, 1H, H-3), 7.66 (d, J = 8.83 Hz, 2H, H-2′, 6′), 7.89 (d, J = 8.83 Hz, 2H, H-3′, 5′), 10.05 (s, 1H, H-9), 12.35 (br, s, 2H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.91 (s, 1C, C-1) 29.44 (s, 1C, C-2) 52.20 (s, 1C, C-4) 53.97 (s, 1C, C-5) 77.42 (s, 1C, C-3) 79.11 (s, 1C, C-6) 118.80 (s, 2C, C-2′, C-6′) 125.29 (s, 1C, C-4′) 130.78 (s, 2C, C-3′, C-5′) 143.69 (m, 1C, C-1′) 167.32 (m, 1C, C-7′) 170.32 (s, 1C, C-8) 172.65 (s, 1C, C-7); HR-MS (ESI): m/z calcd for C15H15NO6Na ([M+Na]+) 328.0797, found 328.0796.
Data for7-l: white solid; yield, 78%; mp: 157–159 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.45–1.69 (m, 4H, H-1, 2), 2.93–2.98 (m, 1H, H-4), 3.02–3.08 (m, 1H, H-5), 4.65 (d, J = 4.10 Hz, 1H, H-6), 4.79 (d, J = 3.78 Hz, 1H, H-3), 7.13 (t, J = 8.83 Hz, 2H, H-2′, 6′), 7.55 (dd, J = 8.83, 5.04 Hz, 2H, H-3′, 5′), 9.73 (s, 1H, H-8), 11.98 (br, s, 1H, H-7); 13C NMR (125 MHz, DMSO-d6) δ: 28.90 (s, 1C, C-1), 29.46 (s, 1C, C-2), 52.15 (s, 1C, C-4), 53.83 (s, 1C, C-5), 77.34 (s, 1C, C-3), 79.10 (s, 2C, C-6), 115.49 (s, 2C, C-3′, C-5′), 121.37 (s, 2C, C-2′, C-6′), 136.13 (s, 1C, C-1′), 157.37 (s, 1C, C-4′), 169.71 (s, 1C, C-8), 172.68 (s, 1C, C-7); HR-MS (ESI): m/z calcd for C14H14FNO4Na ([M+Na]+) 302.0805, found 302.0813.
Data for7-m: yellow solid; yield, 47%; mp: 170–171 °C; 1H NMR (500 MHz, DMSO-d6) δ: 1.46–1.69 (m, 4H, H-1, 2), 2.95–3.00 (m, 1H, H-4), 3.07 (d, J = 9.77 Hz, 1H, H-5), 4.67 (d, J = 4.41 Hz, 1H, H-6), 4.80 (d, J = 3.78 Hz, 1H, H-3), 7.30 (d, J = 8.83 Hz, 2H, H-3′, 5′), 7.65 (d, J = 8.83 Hz, 2H, H-2′, 6′), 9.91 (s, 1H, H-9), 12.01 (s, 1H, H-10); 13C NMR (125 MHz, DMSO-d6) δ: 28.84 (m, 1C, C-1), 29.44 (m, 1C, C-2), 52.17 (m, 1C, C-4), 53.89 (m, 1C, C-5), 77.34 (m, 1C, C-3), 79.07 (m, 1C, C-6), 120.88 (s, 2C, C-3′, C-5′), 121.65 (s, 1C, C-7′), 121.97 (s, 2C, C-2′, C-6′), 138.91 (m, 1C, C-1′), 143.80 (m, 1C, C-4′), 169.85 (m, 1C, C-8), 172.47 (m, 1C, C-7); HR-MS (ESI): m/z calcd for C15H14F3NO5Na ([M+Na]+) 368.0722, found 368.0732.