Shear Stress Inhibits Apoptosis of Ischemic Brain Microvascular Endothelial Cells
Abstract
:1. Introduction
2. Results and Discussion
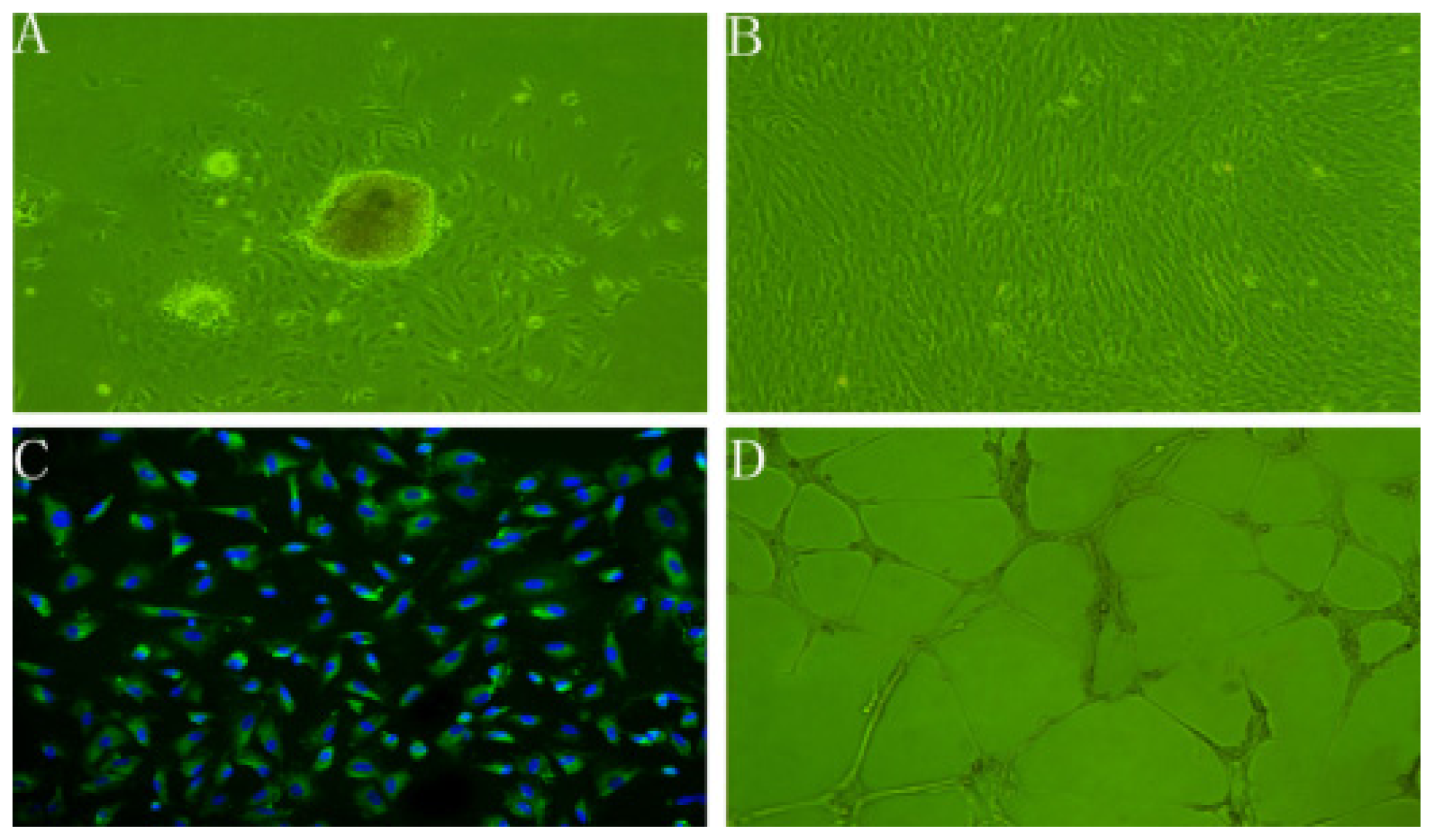
2.1. Characteristics of rBMECs
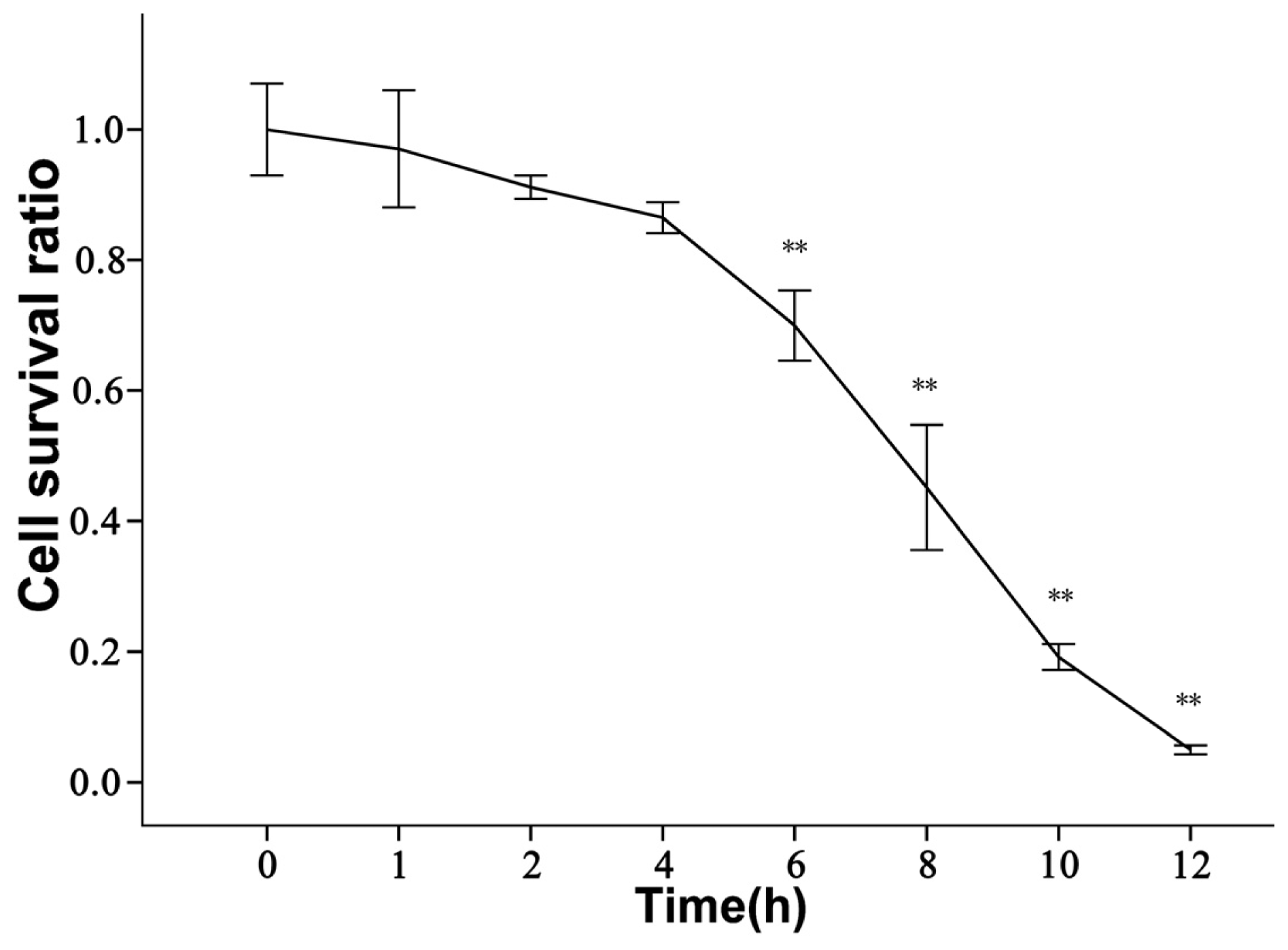
2.2. rBMECs under Ischemic Condition
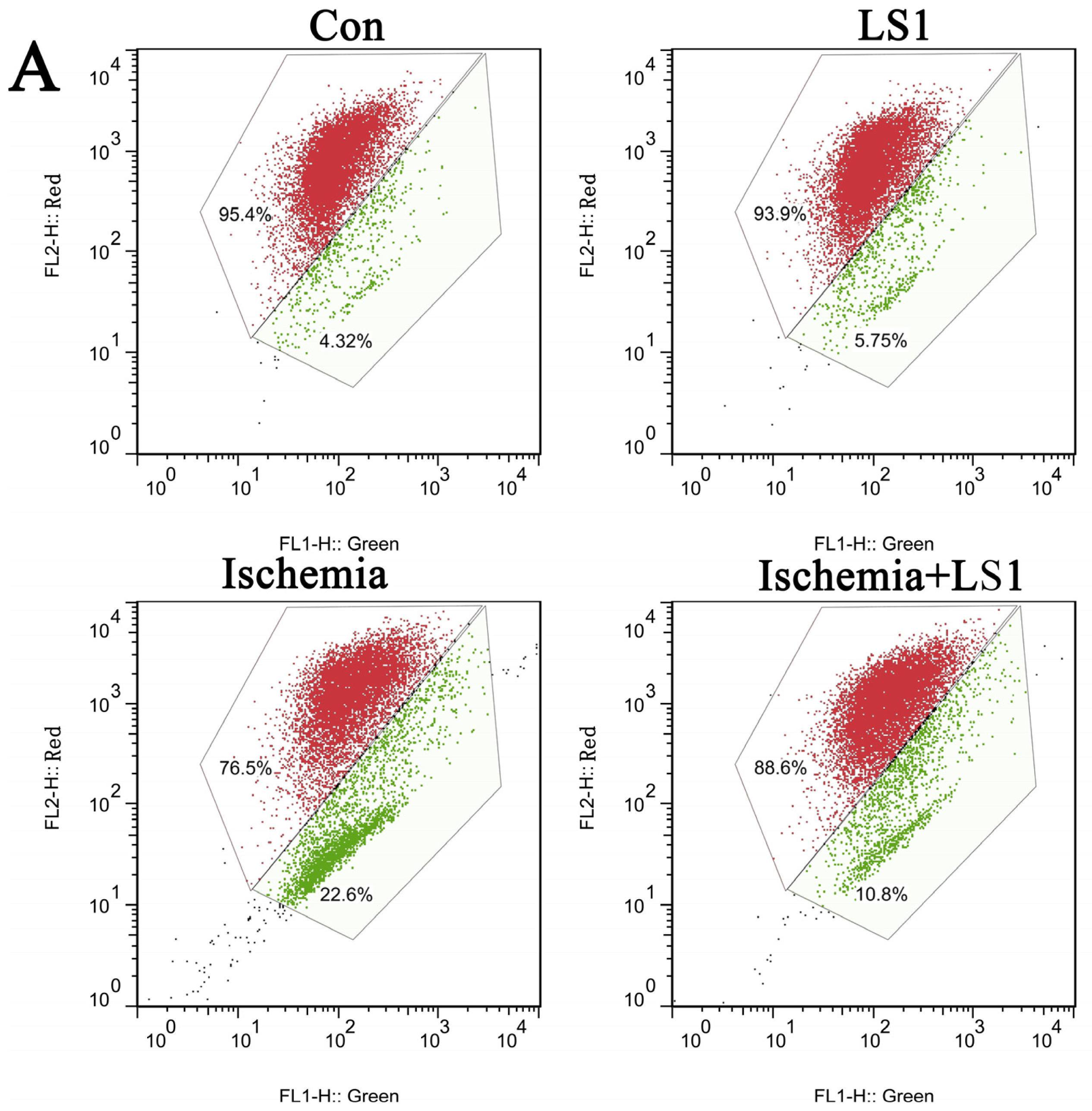
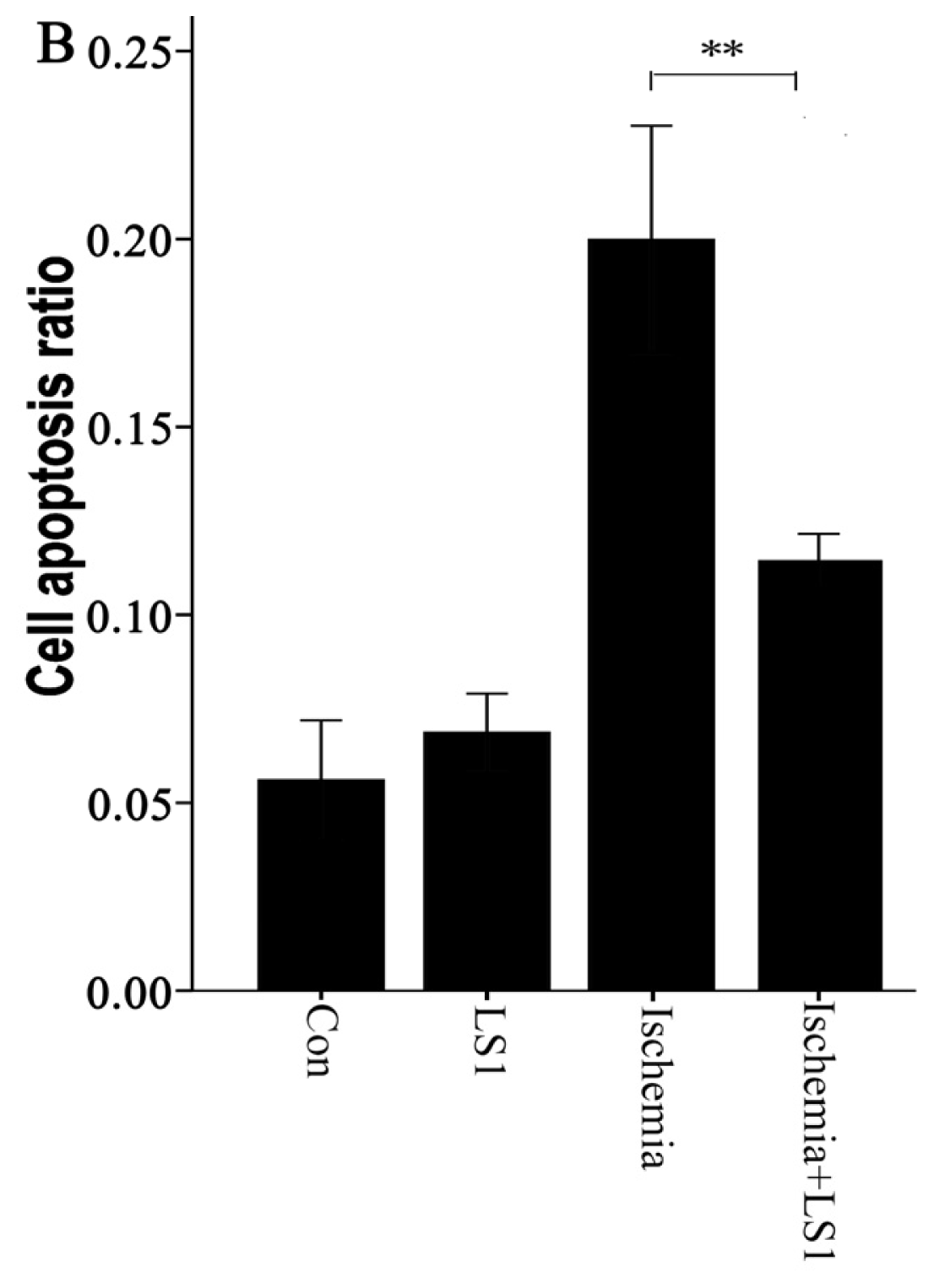
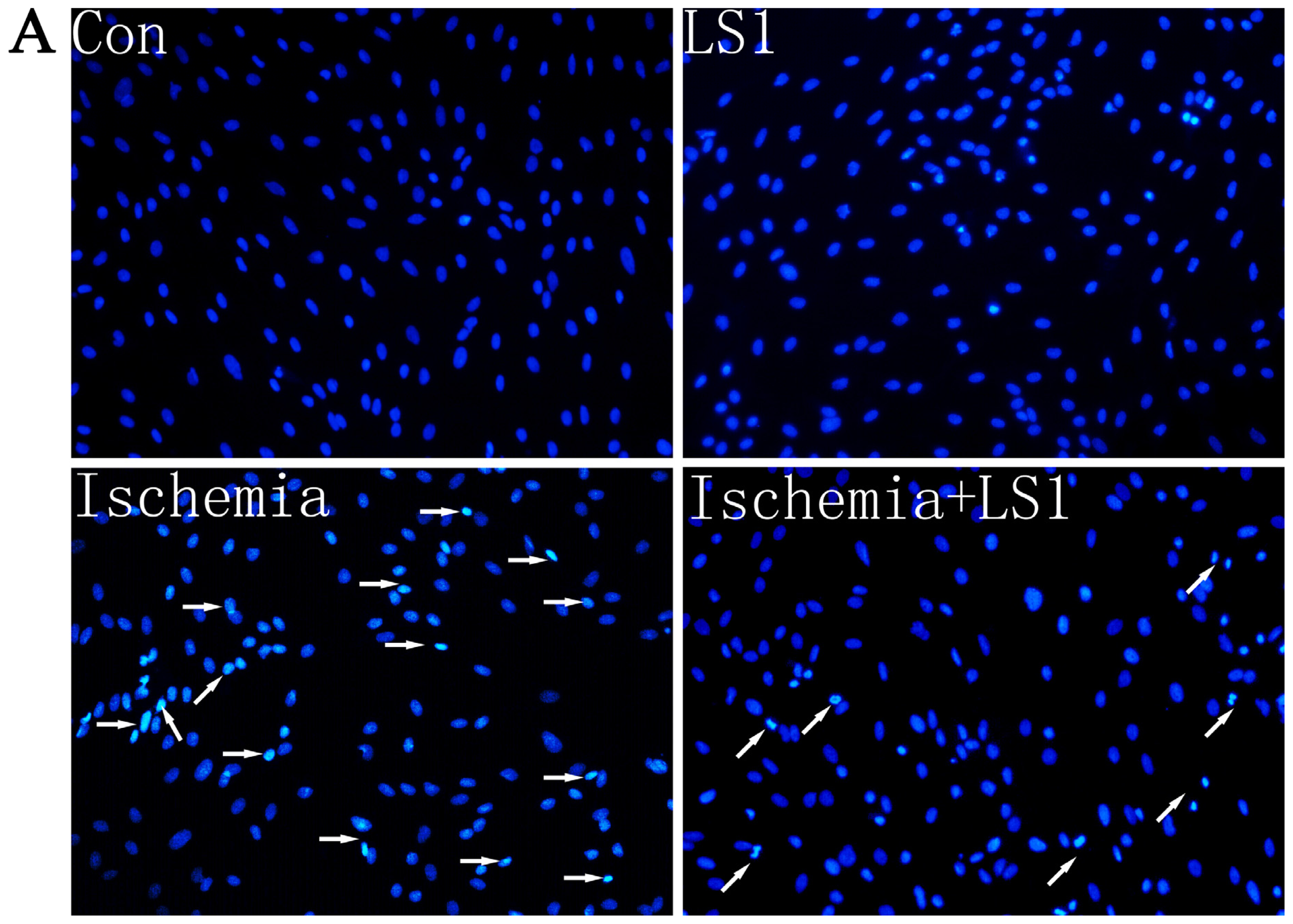
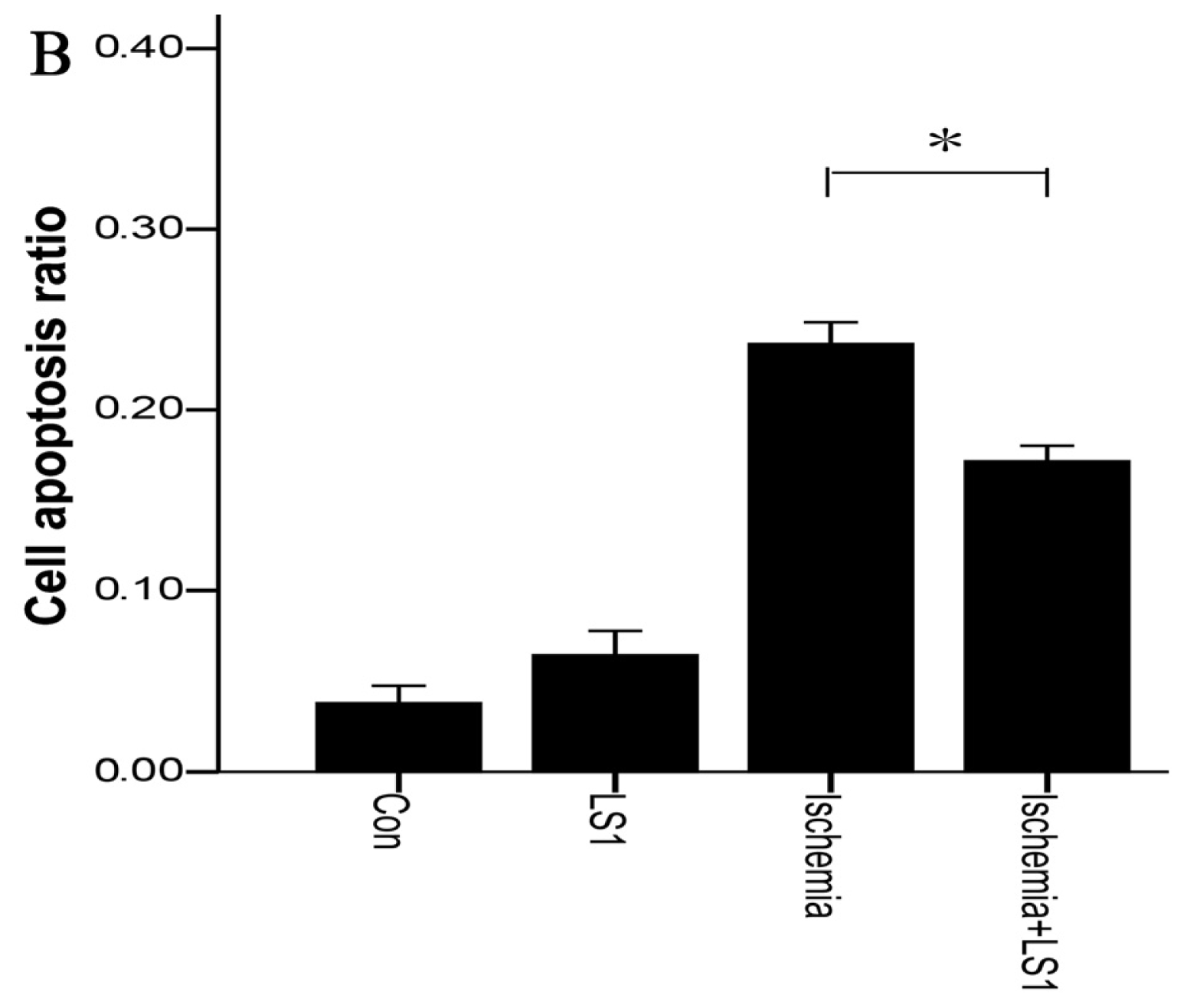
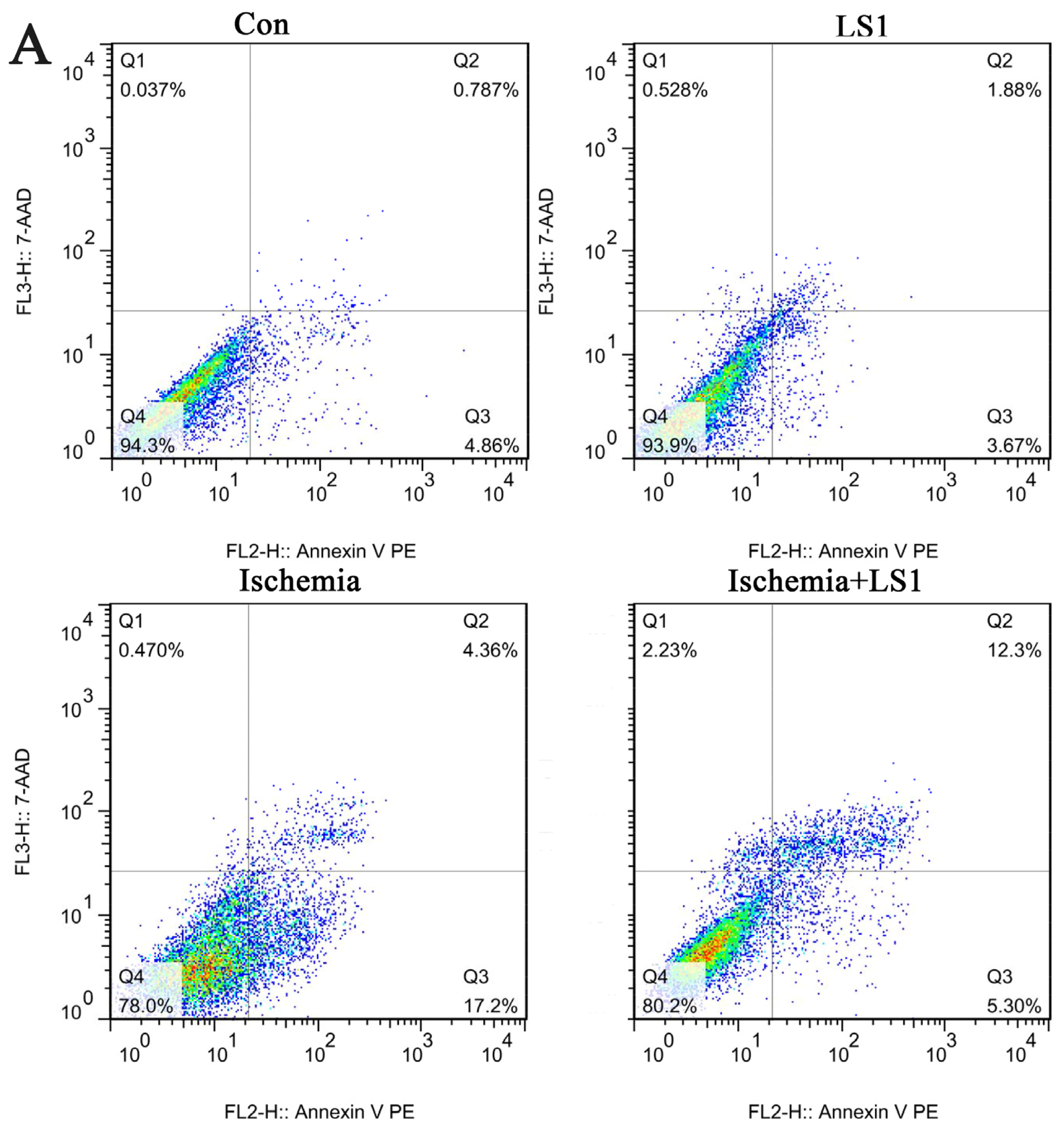
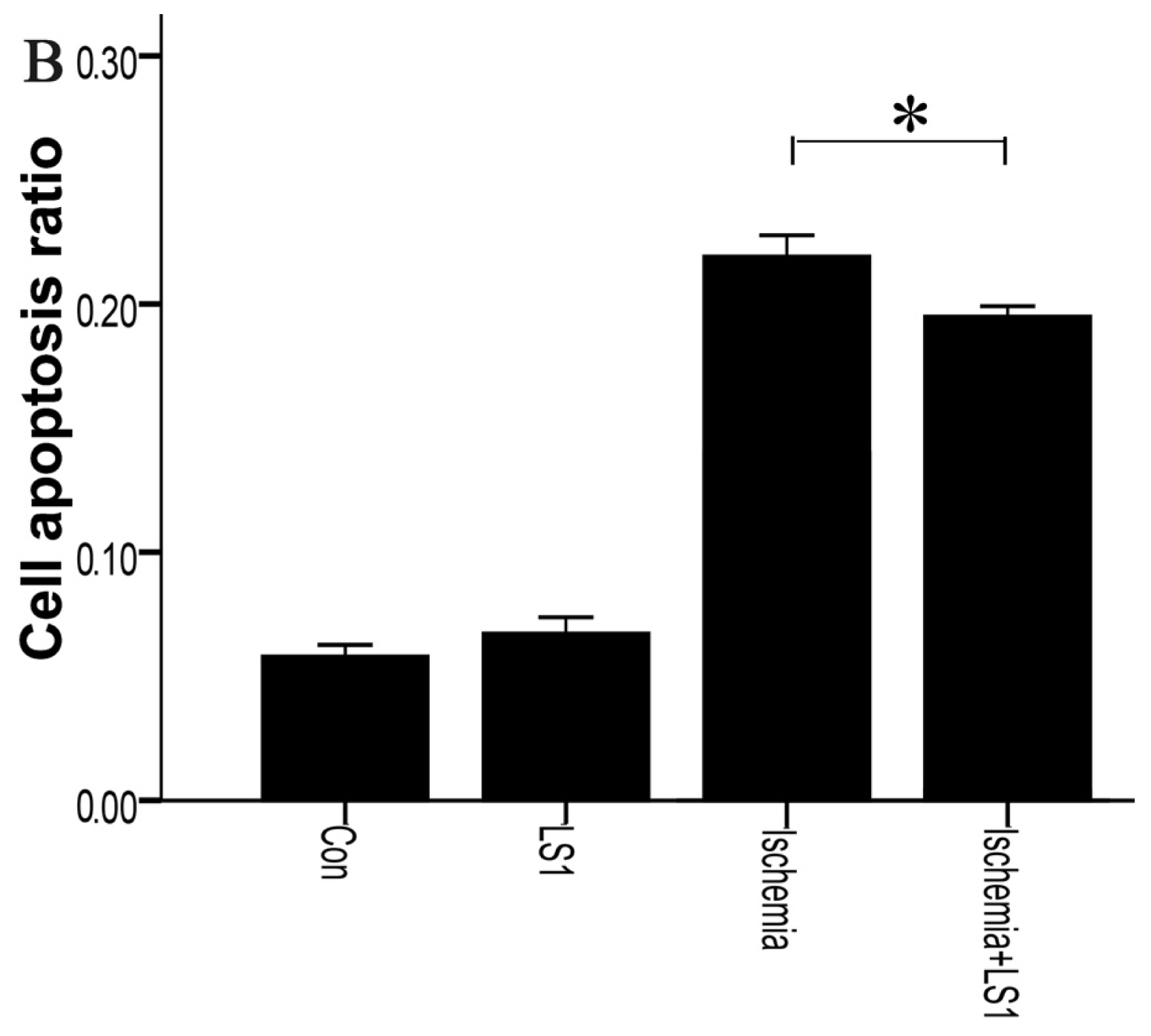
2.3. The LS Effects on Ischemic Cells with PE Annexin V/7-AAD, JC-1 and Hoechst33258 Staining
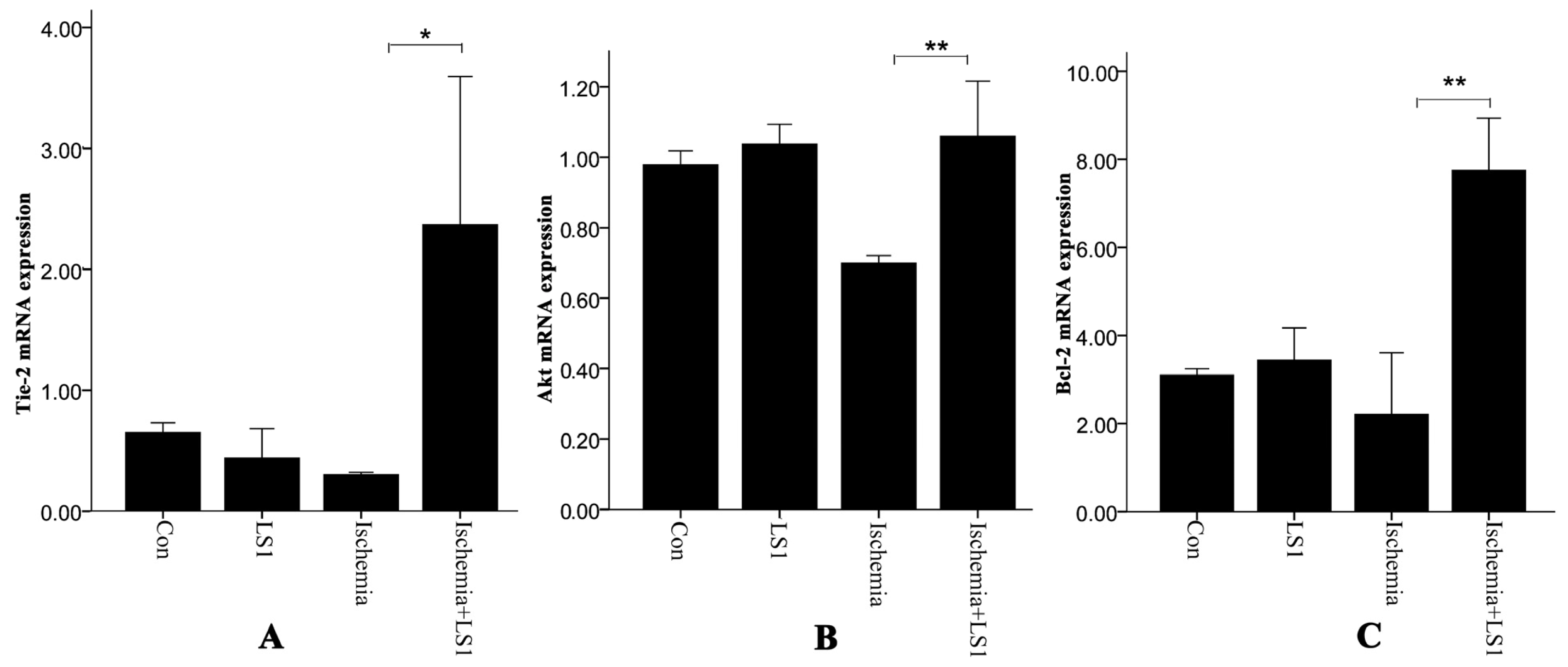
2.4. Expression of Antiapoptotic Tie-2, Akt and Bcl-2
2.5. Discussion
3. Experimental Section
3.1. Reagents
3.2. Isolation and Culture of rBMECs
3.3. Immunocytochemistry
3.4. In vitro Angiogenesis Assay
3.5. Cell Treatments with Ischemia and LS
3.6. WST-8 Assay
3.7. Hoechst 33258 Apoptotic Staining
3.8. Mitochondrial Membrane Potentials Assay
3.9. PE Annexin V/7-AAD Apoptotic Analysis
3.10. Real-Time PCR
3.11. Western Blot
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Sutherland, B.A.; Papadakis, M.; Chen, R.-L.; Buchan, A.M. Cerebral blood flow alteration in neuroprotection following cerebral ischaemia. J. Physiol. Lond 2011, 589, 4105–4114. [Google Scholar]
- Matuszewski, K.A.; Seeger, J.D. Thrombolytic therapy in the treatment of acute ischemic stroke. J. Pharm. Technol 1998, 14, 101–105. [Google Scholar]
- Ivey, F.M.; Ryan, A.S.; Hafer-Macko, C.E.; Macko, R.F. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke 2011, 42, 1994–2000. [Google Scholar]
- Zigmond, M.; Smeyne, R.J. Foreword: Exercise and the brain. Brain Res 2010, 1341, 1–2. [Google Scholar]
- Eames, P.J.; Blake, M.J.; Dawson, S.L.; Panerai, R.B.; Potter, J.F. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2002, 72, 467–472. [Google Scholar]
- Duncker, D.J.; Bache, R.J. Regulation of coronary blood flow during exercise. Physiol. Rev 2008, 88, 1009–1086. [Google Scholar]
- Endres, M.; Gertz, K.; Lindauer, U.; Katchanov, J.; Schultze, J.; Schrock, H.; Nickenig, G.; Kuschinsky, W.; Dirnagl, U.; Laufs, U. Mechanisms of stroke protection by physical activity. Ann. Neurol 2003, 54, 582–590. [Google Scholar]
- Gertz, K.; Priller, J.; Kronenberg, G.; Fink, K.B.; Winter, B.; Schroeck, H.; Ji, S.; Milosevic, M.; Harms, C.; Bohm, M.; et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ. Res 2006, 99, 1132–1140. [Google Scholar]
- Nigro, P.; Abe, J.-i.; Berk, B.C. Flow shear stress and atherosclerosis: A matter of site specificity. Antioxid. Redox Signal 2011, 15, 1405–1414. [Google Scholar]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J 2006, 20, 811–827. [Google Scholar]
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631. [Google Scholar]
- Chan, D.D.; van Dyke, W.S.; Bahls, M.; Connell, S.D.; Critser, P.; Kelleher, J.E.; Kramer, M.A.; Pearce, S.M.; Sharma, S.; Neu, C.P. Mechanostasis in apoptosis and medicine. Prog. Biophys. Mol. Biol 2011, 106, 517–524. [Google Scholar]
- Graf, R.; Apenberg, S.; Freyberg, M.; Friedl, P. A common mechanism for the mechanosensitive regulation of apoptosis in different cell types and for different mechanical stimuli. Apoptosis 2003, 8, 531–538. [Google Scholar]
- Fisher, M. Injuries to the vascular endothelium: Vascular wall and endothelial dysfunction. Rev. Neurol. Dis 2008, 5, 4–11. [Google Scholar]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci 2003, 4, 399–415. [Google Scholar]
- Anderson, D.E.J.; Hinds, M.T. Endothelial cell micropatterning: Methods, effects and applications. Ann. Biomed. Eng 2011, 39, 2329–2345. [Google Scholar]
- Mattiussi, S.; Lazzari, C.; Truffa, S.; Antonini, A.; Soddu, S.; Capogrossi, M.C.; Gaetano, C. Homeodomain interacting protein kinase 2 activation compromises endothelial cell response to laminar flow: Protective role of p21waf1,cip1,sdi1. PLoS One 2009, 4, 1–10. [Google Scholar]
- Metaxa, E.; Meng, H.; Kaluvala, S.R.; Szymanski, M.P.; Paluch, R.A.; Kolega, J. Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am. J. Physiol. Heart Circ. Physiol 2008, 295, 736–742. [Google Scholar]
- Petit, P.X.; Susin, S.A.; Zamzami, N.; Mignotte, B.; Kroemer, G. Mitochondria and programmed cell death: Back to the future. FEBS Lett 1996, 396, 7–13. [Google Scholar]
- Smiley, S.T.; Reers, M.; Mottolahartshorn, C.; Lin, M.; Chen, A.; Smith, T.W.; Steele, G.D.; Chen, L.B. Intracellular heterogeneity in mitochondrial-membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3671–3675. [Google Scholar]
- Elstein, K.H.; Zucker, R.M. Comparison of cellular and nuclear-flow cytometric techniques for discriminating apoptotic subpopulations. Exp. Cell Res 1994, 211, 322–331. [Google Scholar]
- Herault, O.; Colombat, P.; Domenech, J.; Degenne, M.; Bremond, J.L.; Sensebe, L.; Bernard, M.C.; Binet, C. A rapid single-laser flow cytometric method for discrimination of early apoptotic cells in a heterogenous cell population. Br. J. Haematol 1999, 104, 530–537. [Google Scholar]
- Brindle, N.P.J.; Saharinen, P.; Alitalo, K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 2006, 98, 1014–1023. [Google Scholar]
- Peters, K.G.; Kontos, C.D.; Lin, P.C.; Wong, A.L.; Rao, P.; Huang, L.W.; Dewhirst, M.W.; Sankar, S. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog. Horm. Res 2004, 59, 51–71. [Google Scholar]
- Hayes, A.J.; Huang, W.Q.; Mallah, J.; Yang, D.J.; Lippman, M.E.; Li, L.Y. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc. Res 1999, 58, 224–237. [Google Scholar]
- Harfouche, R.; Hassessian, H.M.; Guo, Y.; Faivre, V.; Srikant, C.B.; Yancopoulos, G.D.; Hussain, S.N.A. Mechanisms, which mediate the antiapoptotic effects of angiopoietin-1 on endothelial cells. Microvasc. Res 2002, 64, 135–147. [Google Scholar]
- Lixing, S.; Hao, D.; Guoming, M.; Genlin, X.; Yanchen, D.; Mingyang, L. An testing apparatus for endothelial cells stress. J. Med. Biomech 2003, 18, 229–233. [Google Scholar]
- Koslow, A.R.; Stromberg, R.R.; Friedman, L.I.; Lutz, R.J.; Hilbert, S.L.; Schuster, P. A flow system for the study of shear forces upon cultured endothelial-cells. J. Biomech. Eng. Trans. Asme 1986, 108, 338–341. [Google Scholar]
- Viggers, R.F.; Wechezak, A.R.; Sauvage, L.R. An apparatus to study the response of cultured endothelium to shear-stress. J. Biomech. Eng. Trans. Asme 1986, 108, 332–337. [Google Scholar]
- Abbott, N.J.; Hughes, C.C.W.; Revest, P.A.; Greenwood, J. Development and characterization of a rat-brain capillary endothelial culture—Towards an in vitro blood-brain-barrier. J. Cell Sci 1992, 103, 23–37. [Google Scholar]
- Goldstein, G.W.; Wolinsky, J.S.; Csejtey, J.; Diamond, I. Isolation of metabolically active capillaries from rat-brain. J. Neurochem 1975, 25, 715–717. [Google Scholar]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis 2009, 12, 267–274. [Google Scholar]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun 1999, 36, 47–50. [Google Scholar]
- Lecht, S.; Arien-Zakay, H.; Marcinkiewicz, C.; Lelkes, P.I.; Lazarovici, P. Nerve growth factor-induced protection of brain capillary endothelial cells exposed to oxygen-glucose deprivation involves attenuation of erk phosphorylation. J. Mol. Neurosci 2010, 41, 183–192. [Google Scholar]
- James, J.L.; Whitley, G.S.; Cartwright, J.E. Shear stress and spiral artery remodelling: The effects of low shear stress on trophoblast-induced endothelial cell apoptosis. Cardiovasc Res 2011, 90, 130–139. [Google Scholar]
- DeBusk, L.M.; Hallahan, D.E.; Lin, P.C. Akt is a major angiogenic mediator downstream of the Ang1/Tie2 signaling pathway. Exp. Cell Res 2004, 298, 167–177. [Google Scholar]
- Papapetropoulos, A.; Fulton, D.; Mahboubi, K.; Kalb, R.G.; O’Connor, D.S.; Li, F.Z.; Altieri, D.C.; Sessa, W.C. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J. Biol. Chem 2000, 275, 9102–9105. [Google Scholar]
- Kim, I.; Kim, H.G.; So, J.N.; Kim, J.H.; Kwak, H.J.; Koh, G.Y. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ. Res 2000, 86, 24–29. [Google Scholar]
- Guo, Q.; Jin, J.; Yuan, J.X.J.; Zeifman, A.; Chen, J.; Shen, B.; Huang, J. VEGF, Bcl-2 and Bad regulated by angiopoietin-1 in oleic acid induced acute lung injury. Biochem. Biophys. Res. Commun 2011, 413, 630–636. [Google Scholar]
- Kumar, P.; Miller, A.I.; Polverini, P.J. p38 MAPK mediates gamma-irradiation-induced endothelial cell apoptosis and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J. Biol. Chem 2004, 279, 43352–43360. [Google Scholar]

© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tian, S.; Bai, Y.; Yang, L.; Wang, X.; Wu, Y.; Jia, J.; Zhu, Y.; Cheng, Y.; Zhang, P.; Wu, J.; et al. Shear Stress Inhibits Apoptosis of Ischemic Brain Microvascular Endothelial Cells. Int. J. Mol. Sci. 2013, 14, 1412-1427. https://doi.org/10.3390/ijms14011412
Tian S, Bai Y, Yang L, Wang X, Wu Y, Jia J, Zhu Y, Cheng Y, Zhang P, Wu J, et al. Shear Stress Inhibits Apoptosis of Ischemic Brain Microvascular Endothelial Cells. International Journal of Molecular Sciences. 2013; 14(1):1412-1427. https://doi.org/10.3390/ijms14011412
Chicago/Turabian StyleTian, Shan, Yulong Bai, Lin Yang, Xinggang Wang, Yi Wu, Jie Jia, Yulian Zhu, Yong Cheng, Pengyue Zhang, Junfa Wu, and et al. 2013. "Shear Stress Inhibits Apoptosis of Ischemic Brain Microvascular Endothelial Cells" International Journal of Molecular Sciences 14, no. 1: 1412-1427. https://doi.org/10.3390/ijms14011412




