Anthocyanins Downregulate Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglial Cells by Suppressing the NF-κB and Akt/MAPKs Signaling Pathways
Abstract
:1. Introduction
2. Results and Discussion
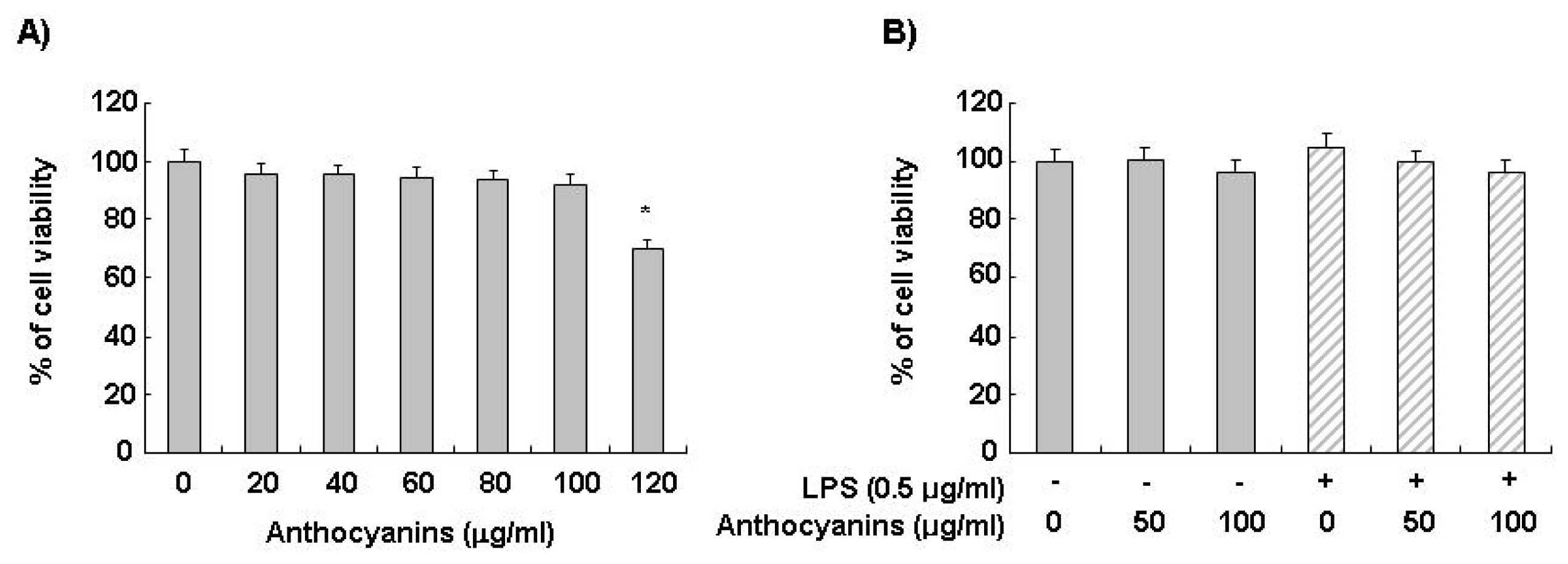
2.1. Effects of Anthocyanins and LPS on BV2 Cell Viability
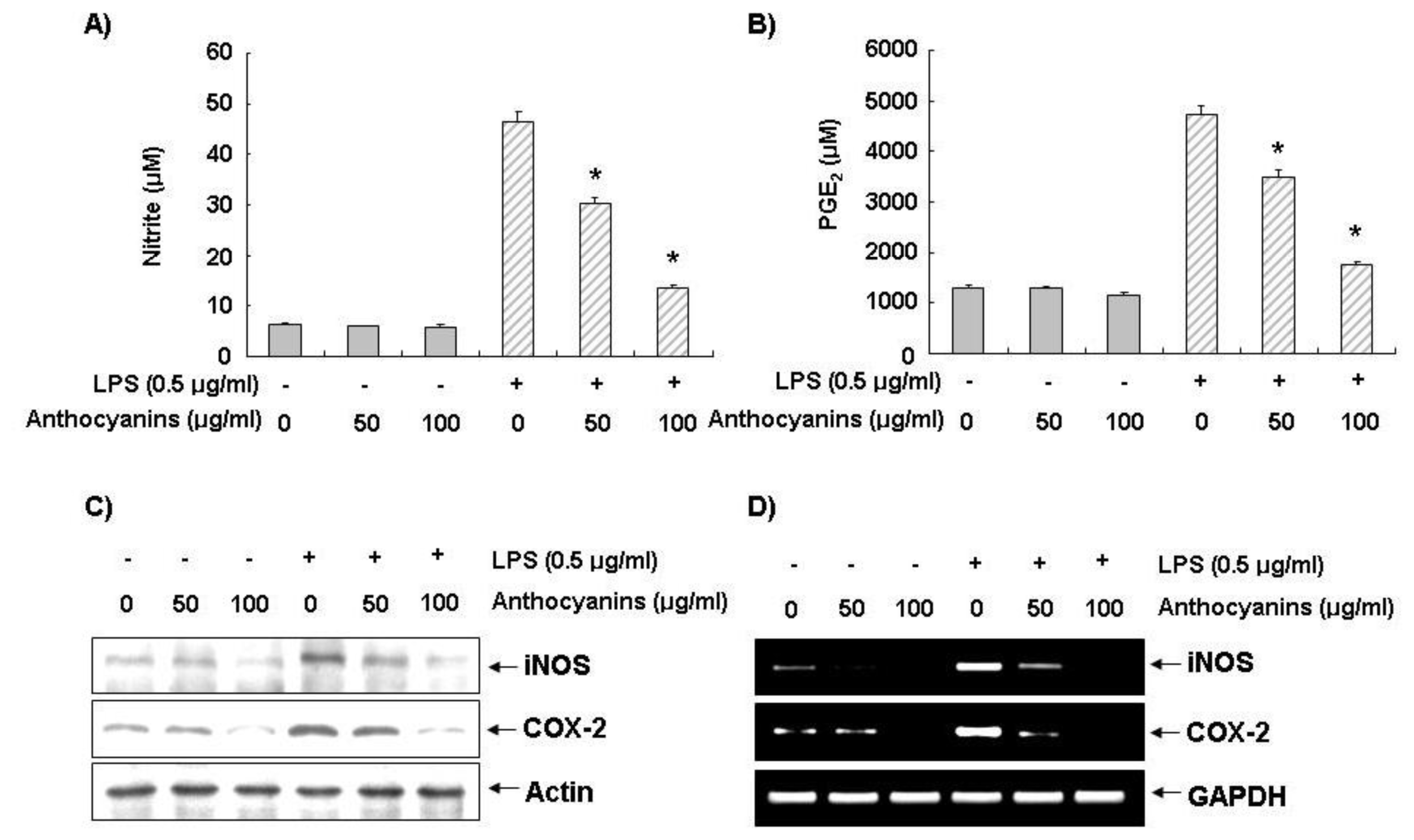
2.2. Effects of Anthocyanins on LPS-Induced NO and PGE2 Production in BV2 Cells
2.3. Effects of Anthocyanins on LPS-Induced iNOS and COX-2 Expression in BV2 Cells
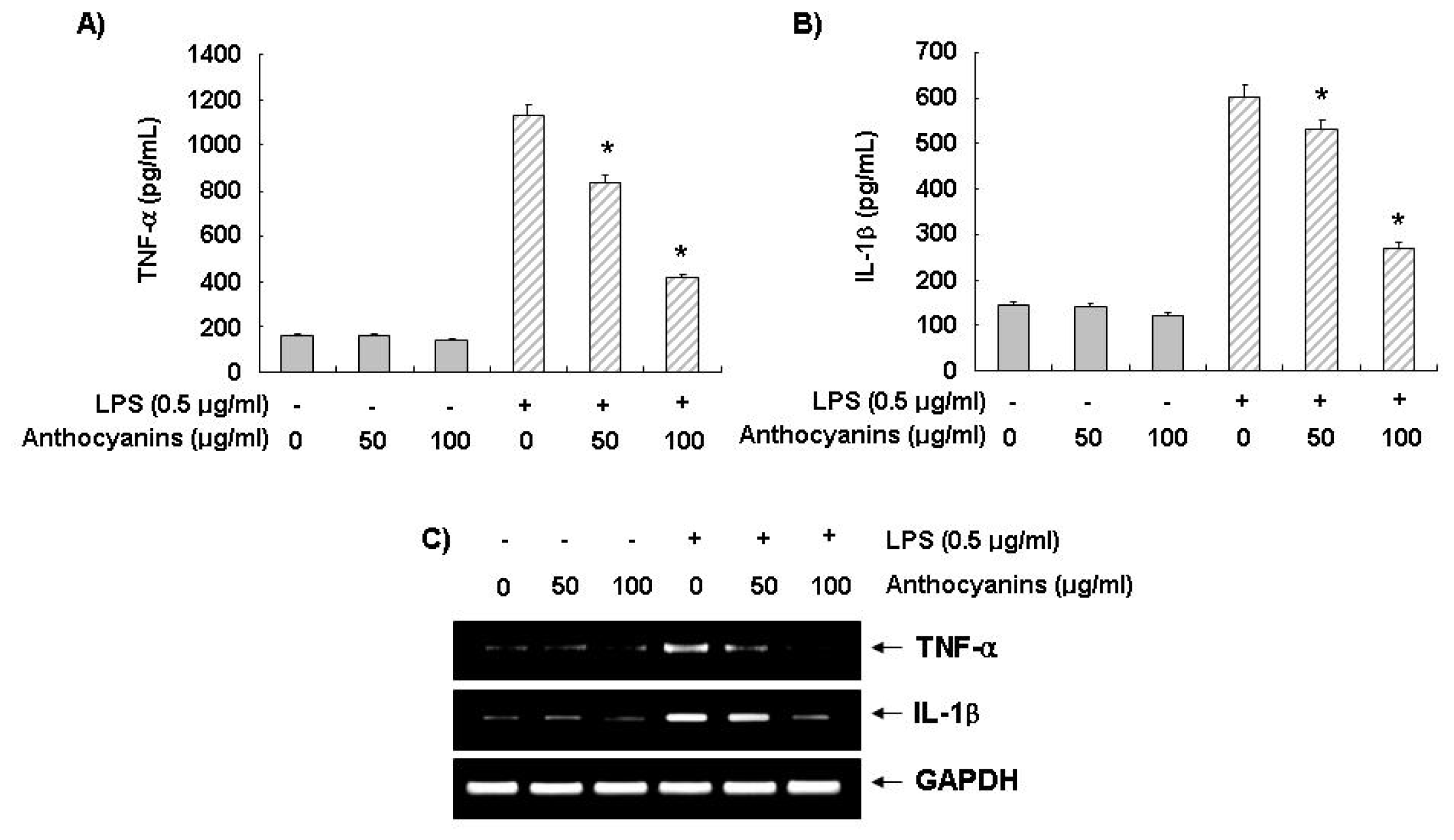
2.4. Effects of Anthocyanins on LPS-Induced TNF-α and IL-1β Production and Expression
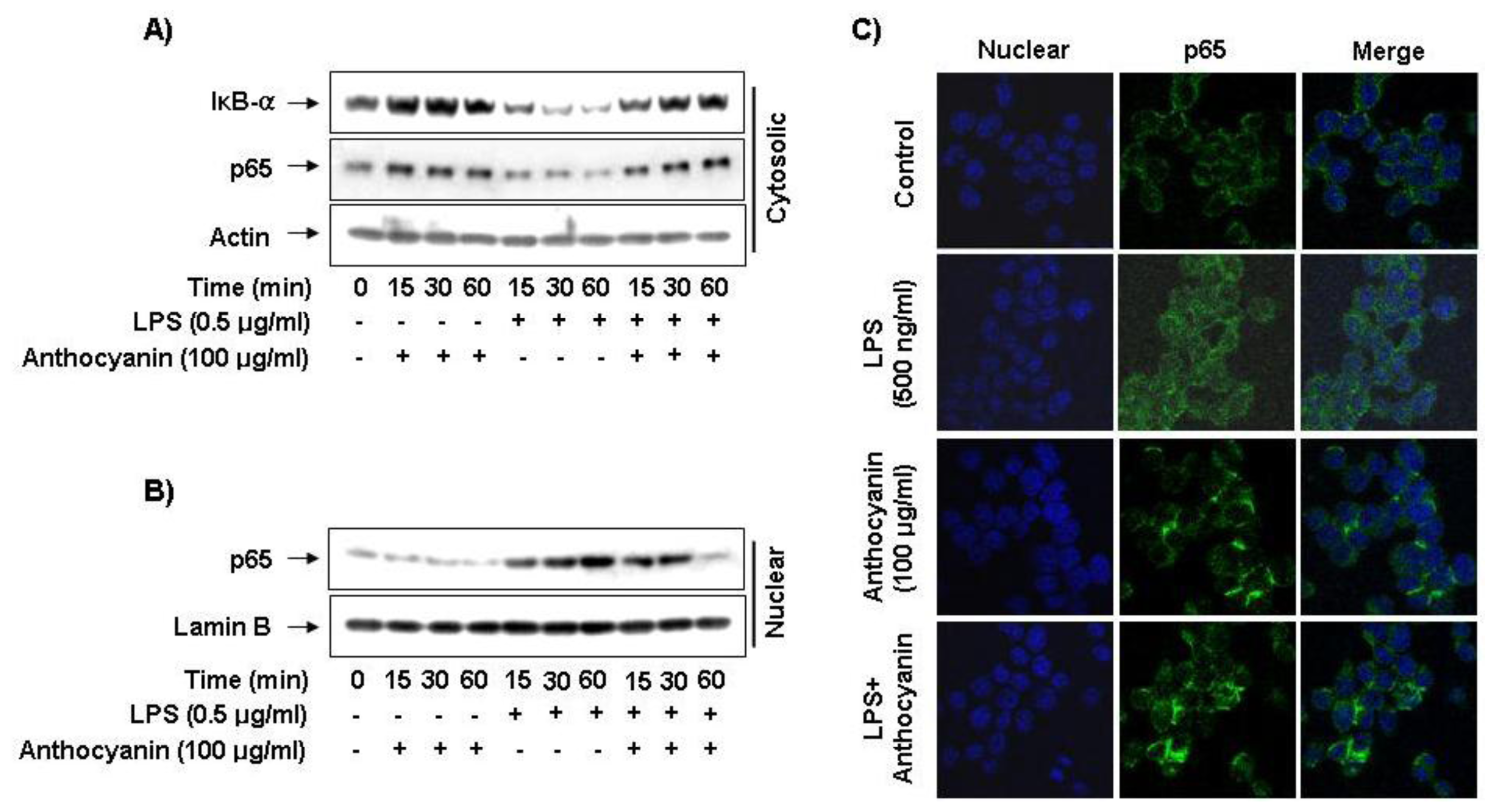
2.5. Effect of Anthocyanins on NF-κB Activity in LPS-Induced BV2 Microglia
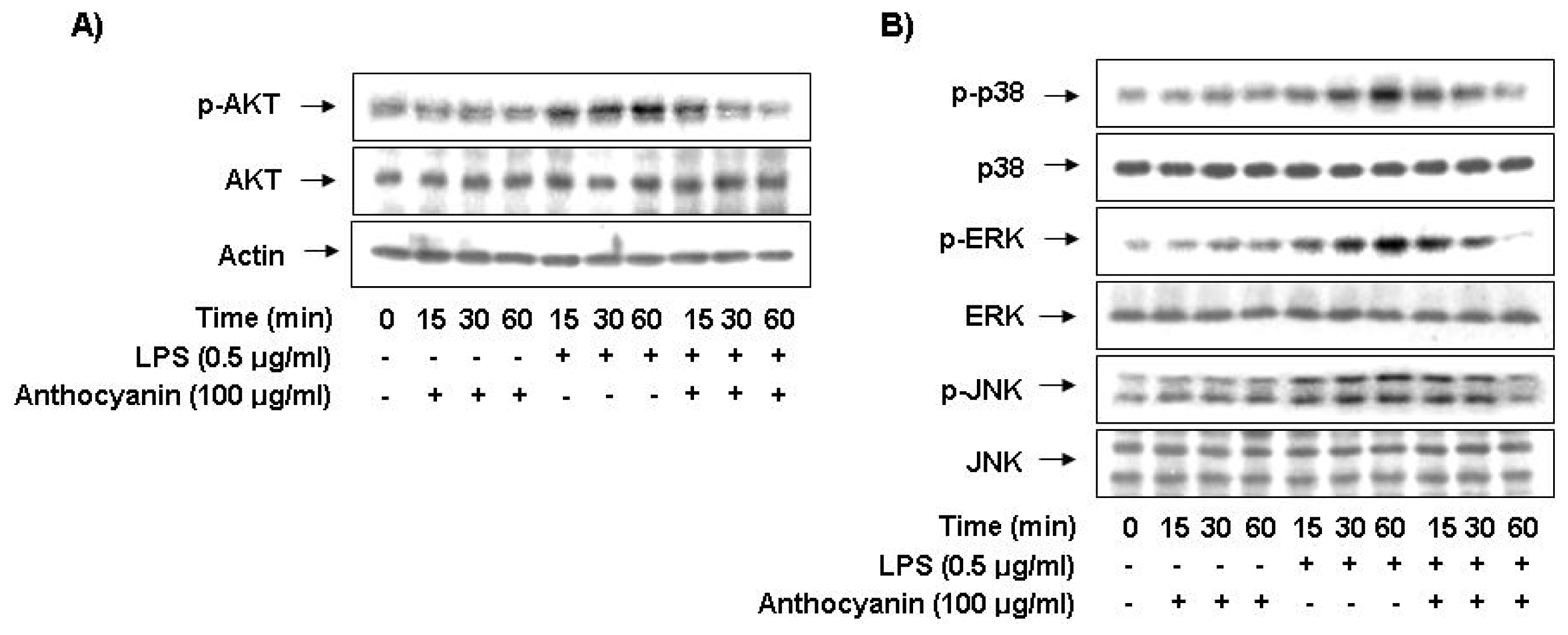
2.6. Anthocyanins Inhibits LPS-Stimulated Phosphorylation of Akt and MAPKs in BV2 Microglia
2.7. Discussion
3. Experimental Section
3.1. Reagents and Antibodies
3.2. Preparation of Anthocyanins
3.3. Cell Culture and Cell Viability
3.4. Nitrite Assay
3.5. Measurement of PGE2 Production
3.6. Measurement of IL-1β and TNF-α Production
3.7. Isolation of Total RNA and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
3.8. Western Blot Analysis
3.9. Immunofluorescence Analysis
3.10. Statistical Analyses
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cheeran, M.; Lokensgard, J.R.; Peterson, P.K. Role of microglia in central nervous system infections. Clin. Microbiol. Rev 2004, 17, 942–964. [Google Scholar]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem 2006, 281, 21362–21368. [Google Scholar]
- Stone, D.K.; Reynolds, A.D.; Mosley, R.L.; Gendelman, H.E. Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid. Redox Signal 2009, 11, 2151–2166. [Google Scholar]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev 2007, 87, 315–424. [Google Scholar]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar]
- Hou, D.X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med 2003, 3, 149–159. [Google Scholar]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res 2006, 40, 1014–1028. [Google Scholar]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett 2008, 269, 281–290. [Google Scholar]
- Thomasset, S.; Teller, N.; Cai, H.; Marko, D.; Berry, D.P.; Steward, W.P.; Gescher, A.J. Do anthocyanins and anthocyanidins, cancer chemopreventive pigments in the diet, merit development as potential drugs? Cancer Chemother. Pharmacol 2009, 64, 201–211. [Google Scholar]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci 2010, 11, 1679–1703. [Google Scholar]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr 2011, 2, 1–7. [Google Scholar]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev 2000, 13, 79–106. [Google Scholar]
- Kang, T.H.; Hur, J.Y.; Kim, H.B.; Ryu, J.H.; Kim, S.Y. Neuroprotective effects of the cyanidin-3-O-β-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Lett 2006, 391, 122–126. [Google Scholar]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res 2007, 51, 675–683. [Google Scholar]
- Varadinova, M.G.; Docheva-Drenska, D.I.; Boyadjieva, N.I. Effects of anthocyanins on learning and memory of ovariectomized rats. Menopause 2009, 16, 345–349. [Google Scholar]
- Chen, G.; Luo, J. Anthocyanins: Are they beneficial in treating ehanol neurotoxicity? Neurotox. Res 2010, 17, 91–101. [Google Scholar]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol 2010, 1, 163–187. [Google Scholar]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res 2012, 56, 159–170. [Google Scholar]
- Kim, H.J.; Tsoy, I.; Park, J.M.; Chung, J.I.; Shin, S.C.; Chang, K.C. Anthocyanins from soybean seed coat inhibit the expression of TNF-α-induced genes associated with ischemia/reperfusion in endothelial cell by NF-κB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett 2006, 580, 1391–1397. [Google Scholar]
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.I.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res 2009, 53, 1419–1429. [Google Scholar]
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem Toxicol 2009, 47, 2806–2812. [Google Scholar]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Shim, H.J.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; et al. Protective effect of anthocyanins from black soybean seed coats on UVB-induced apoptotic cell death in vitro and in vivo. J. Agric. Food Chem 2008, 56, 10600–10605. [Google Scholar]
- Kim, S.H.; Smith, C.J.; van Eldik, L.J. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1β production. Neurobiol. Aging 2004, 25, 431–439. [Google Scholar]
- Madrid, L.V.; Wang, C.Y.; Guttridge, D.C.; Schottelius, A.J.; Baldwin, A.S., Jr; Mayo, M.W. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 2000, 20, 1626–1638. [Google Scholar]
- Wei, J.; Feng, J. Signaling pathways associated with inflammatory bowel disease. Recent Pat. Inflamm. Allergy Drug Discov 2010, 4, 105–117. [Google Scholar]
- Zhang, Y.; Dong, C. Regulatory mechanisms of mitogen-activated kinase signaling. Cell. Mol. Life Sci 2007, 64, 2771–2289. [Google Scholar]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci 1996, 19, 312–318. [Google Scholar]
- Minghetti, L.; Levi, G. Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Prog. Neurobiol 1998, 54, 99–125. [Google Scholar]
- Weinstein, S.L.; Gold, M.R.; Defranco, A.L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc. Natl. Acad. Sci. USA 1991, 88, 4148–4152. [Google Scholar]
- Rankine, E.L.; Hughes, P.M.; Botham, M.S.; Perry, V.H.; Felton, L.M. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. Eur. J. Neurosci 2006, 24, 77–86. [Google Scholar]
- Lynch, M.A. The multifaceted profile of activated microglia. Mol. Neurobiol 2009, 40, 139–156. [Google Scholar]
- Ohshima, H.; Bartsch, H. Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat. Res 1994, 305, 253–264. [Google Scholar]
- Murphy, S. Production of nitric oxide by glial cells: Regulation and potential roles in the CNS. Glia 2000, 29, 1–13. [Google Scholar]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci 2007, 8, 57–69. [Google Scholar]
- Brown, G.C.; Bal-Price, A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol. Neurobiol 2003, 27, 325–355. [Google Scholar]
- Korotkova, M.; Westman, M.; Gheorghe, K.R.; af Klint, E.; Trollmo, C.; Ulfgren, A.K.; Klareskog, L.; Jakobsson, P.J. Effects of antirheumatic treatments on the prostaglandin E2 biosynthetic pathway. Arthritis Rheum 2005, 52, 3439–3447. [Google Scholar]
- Kawano, T.; Anrather, J.; Zhou, P.; Park, L.; Wang, G.; Frys, K.A.; Kunz, A.; Cho, S.; Orio, M.; Iadecola, C. Prostaglandin E2 EP1 receptors: Downstream effectors of COX-2 neurotoxicity. Nat. Med 2006, 12, 225–229. [Google Scholar]
- Popa, C.; Netea, M.G.; van Riel, P.L.; van der Meer, J.W.; Stalenhoef, A.F. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res 2007, 48, 751–762. [Google Scholar]
- Lee, J.W.; Lee, M.S.; Kim, T.H.; Lee, H.J.; Hong, S.S.; Noh, Y.H.; Hwang, B.Y.; Ro, J.S.; Hong, J.T. Inhibitory effect of inflexinol on nitric oxide generation and iNOS expression via inhibition of NF-κB activation. Mediators Inflamm 2007, 2007, 93148–93157. [Google Scholar]
- Baima, E.T.; Guzova, J.A.; Mathialagan, S.; Nagiec, E.E.; Hardy, M.M.; Song, L.R.; Bonar, S.L.; Weinberg, R.A.; Selness, S.R.; Woodard, S.S.; et al. Novel insights into the cellular mechanisms of the anti-inflammatory effects of NF-κB essential modulator binding domain peptides. J. Biol. Chem 2010, 285, 13498–13506. [Google Scholar]
- Khanjani, S.; Kandola, M.K.; Lindstrom, T.M.; Sooranna, S.R.; Melchionda, M.; Lee, Y.S.; Terzidou, V.; Johnson, M.R.; Bennett, P.R. NF-κB regulation: The nuclear response. J. Cell. Mol. Med 2009, 13, 631–643. [Google Scholar]
- Lee, J.Y.; Jhun, B.S.; Oh, Y.T.; Lee, J.H.; Choe, W.; Baik, H.H.; Ha, J.; Yoon, K.S.; Kim, S.S.; Kang, I. Activation of adenosine A3 receptor suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of PI3-kinase/Akt and NF-κB activation in murine BV2 microglial cells. Neurosci. Lett 2006, 396, 1–6. [Google Scholar]
- Bae, D.S.; Kim, Y.H.; Pan, C.H.; Nho, C.W.; Samdan, J.; Yansan, J.; Lee, J.K. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages. BMB Rep 2012, 45, 108–113. [Google Scholar]
- Lee, Y.A.; Choi, H.M.; Lee, S.H.; Yang, H.I.; Yoo, M.C.; Hong, S.J.; Kim, K.S. Synergy between adiponectin and interleukin-1β on the expression of interleukin-6, interleukin-8, and cyclooxygenase-2 in fibroblast-like synoviocytes. Exp. Mol. Med 2012, 44, 440–447. [Google Scholar]
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jeong, J.-W.; Lee, W.S.; Shin, S.C.; Kim, G.-Y.; Choi, B.T.; Choi, Y.H. Anthocyanins Downregulate Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglial Cells by Suppressing the NF-κB and Akt/MAPKs Signaling Pathways. Int. J. Mol. Sci. 2013, 14, 1502-1515. https://doi.org/10.3390/ijms14011502
Jeong J-W, Lee WS, Shin SC, Kim G-Y, Choi BT, Choi YH. Anthocyanins Downregulate Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglial Cells by Suppressing the NF-κB and Akt/MAPKs Signaling Pathways. International Journal of Molecular Sciences. 2013; 14(1):1502-1515. https://doi.org/10.3390/ijms14011502
Chicago/Turabian StyleJeong, Jin-Woo, Won Sup Lee, Sung Chul Shin, Gi-Young Kim, Byung Tae Choi, and Yung Hyun Choi. 2013. "Anthocyanins Downregulate Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglial Cells by Suppressing the NF-κB and Akt/MAPKs Signaling Pathways" International Journal of Molecular Sciences 14, no. 1: 1502-1515. https://doi.org/10.3390/ijms14011502




