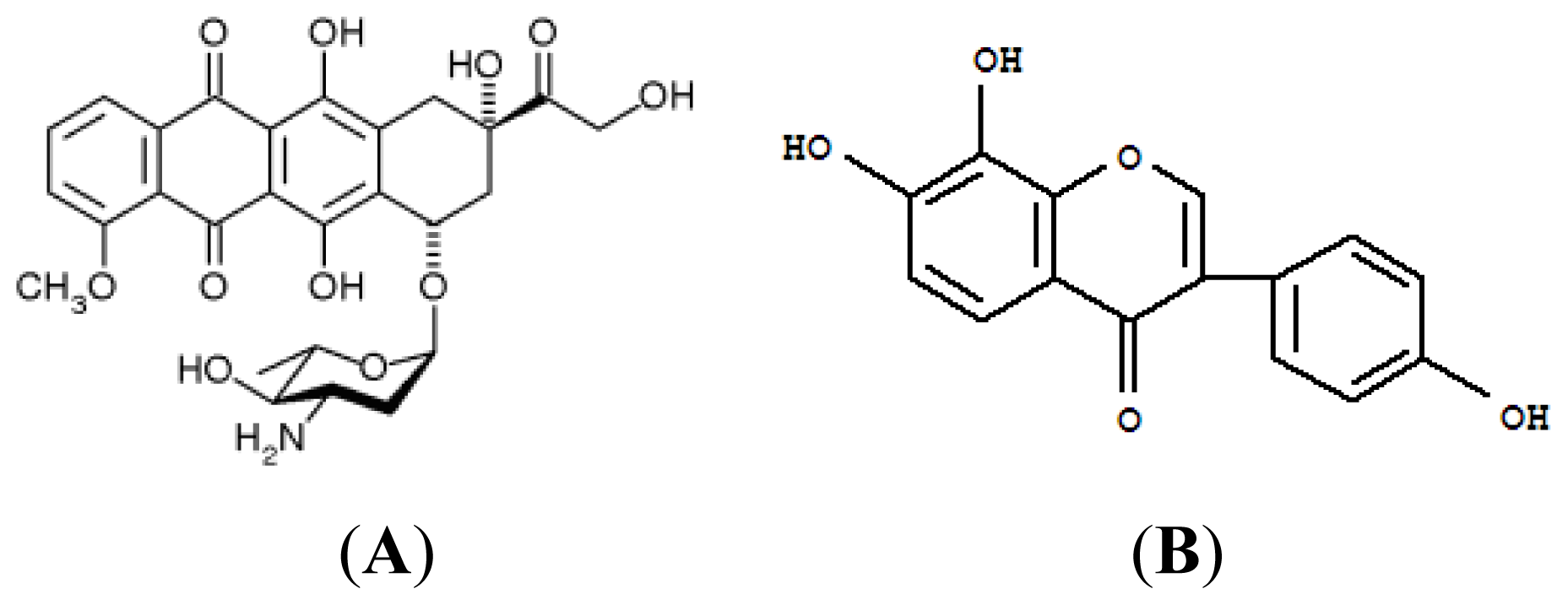
A Potential Daidzein Derivative Enhances Cytotoxicity of Epirubicin on Human Colon Adenocarcinoma Caco-2 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Combined Epi and 8HD Treatment Significantly Increased Epi Cytotoxicity and Caused Synergy
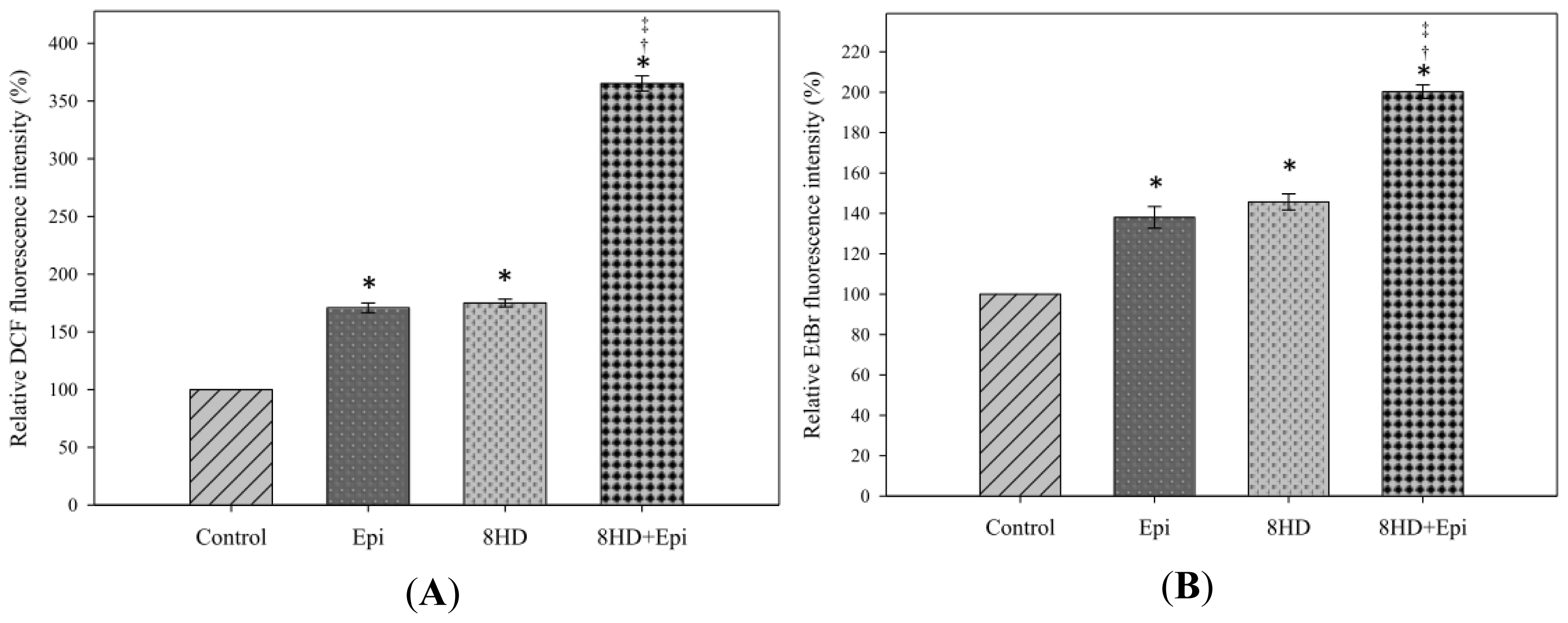
2.1.2. Combined 8HD and Epi Treatment Induced ROS Production
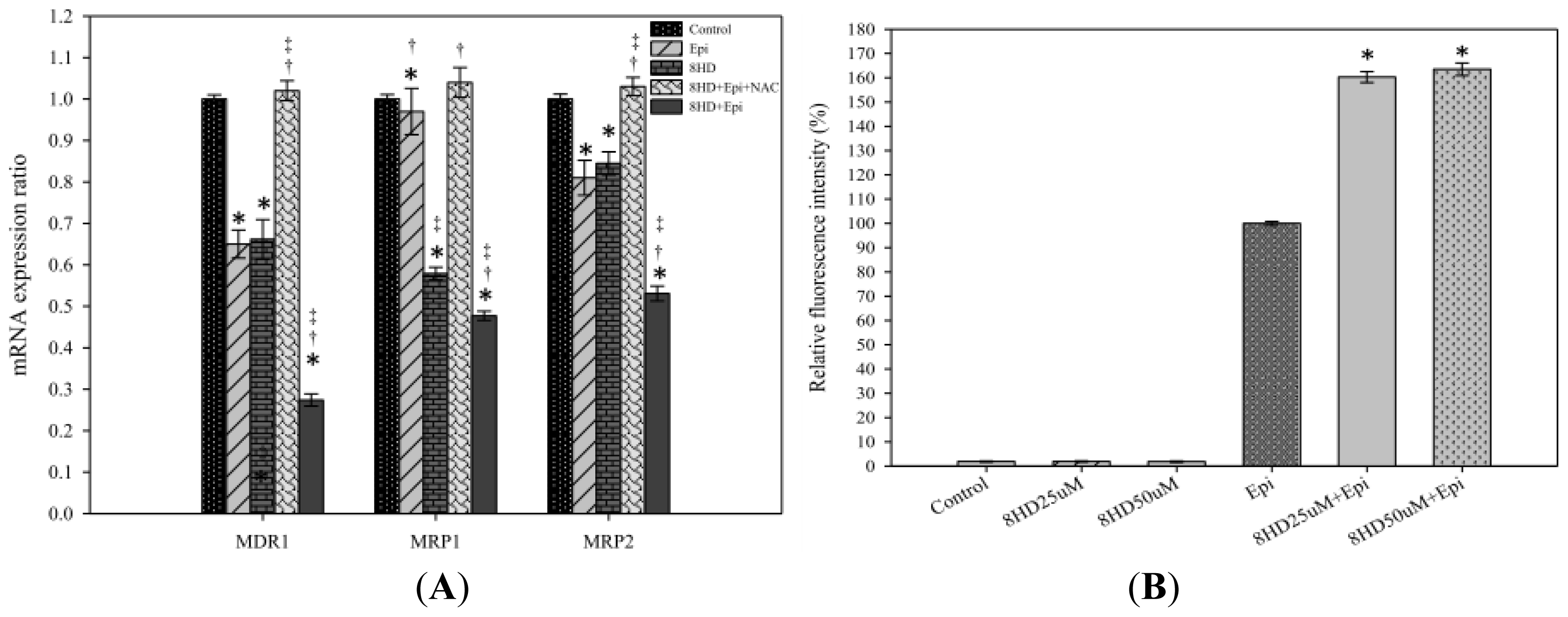
2.1.3. Combined 8HD and Epi Treatment Significantly Inhibited mRNA Expression Levels of P-gp, MRP1, and MRP2
2.1.4. 8HD Significantly Increased the Intracellular Epi Accumulation in Caco-2 Cells
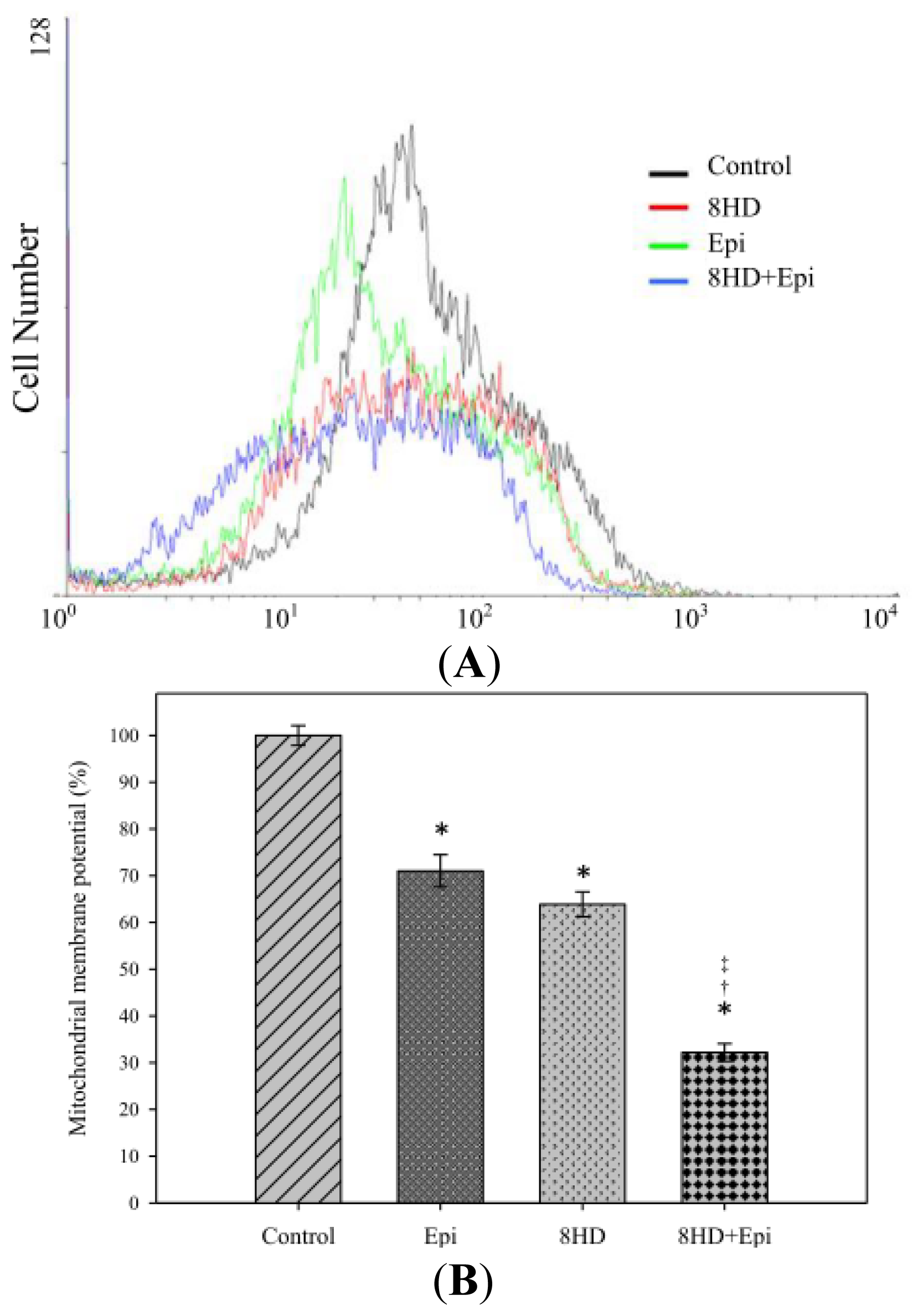
2.1.5. Combined 8HD with Epi Decreased Mitochondrial Membrane Potential
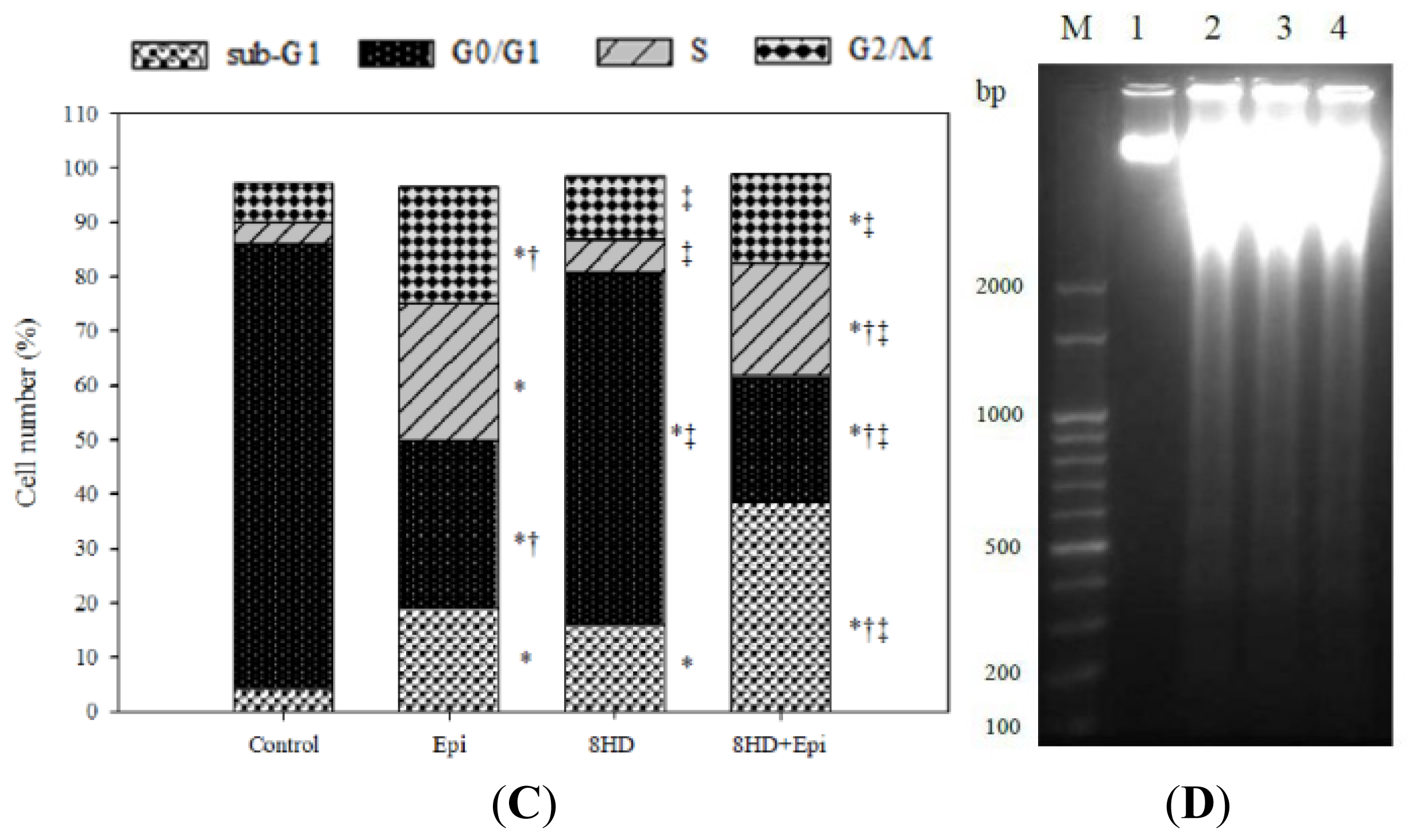
2.1.6. Combined 8HD and Epi Treatment Significantly Increased Sub-G1 Phase of Caco-2 Cell Cycle
2.1.7. 8HD and/or Epi-Induced DNA Fragmentation
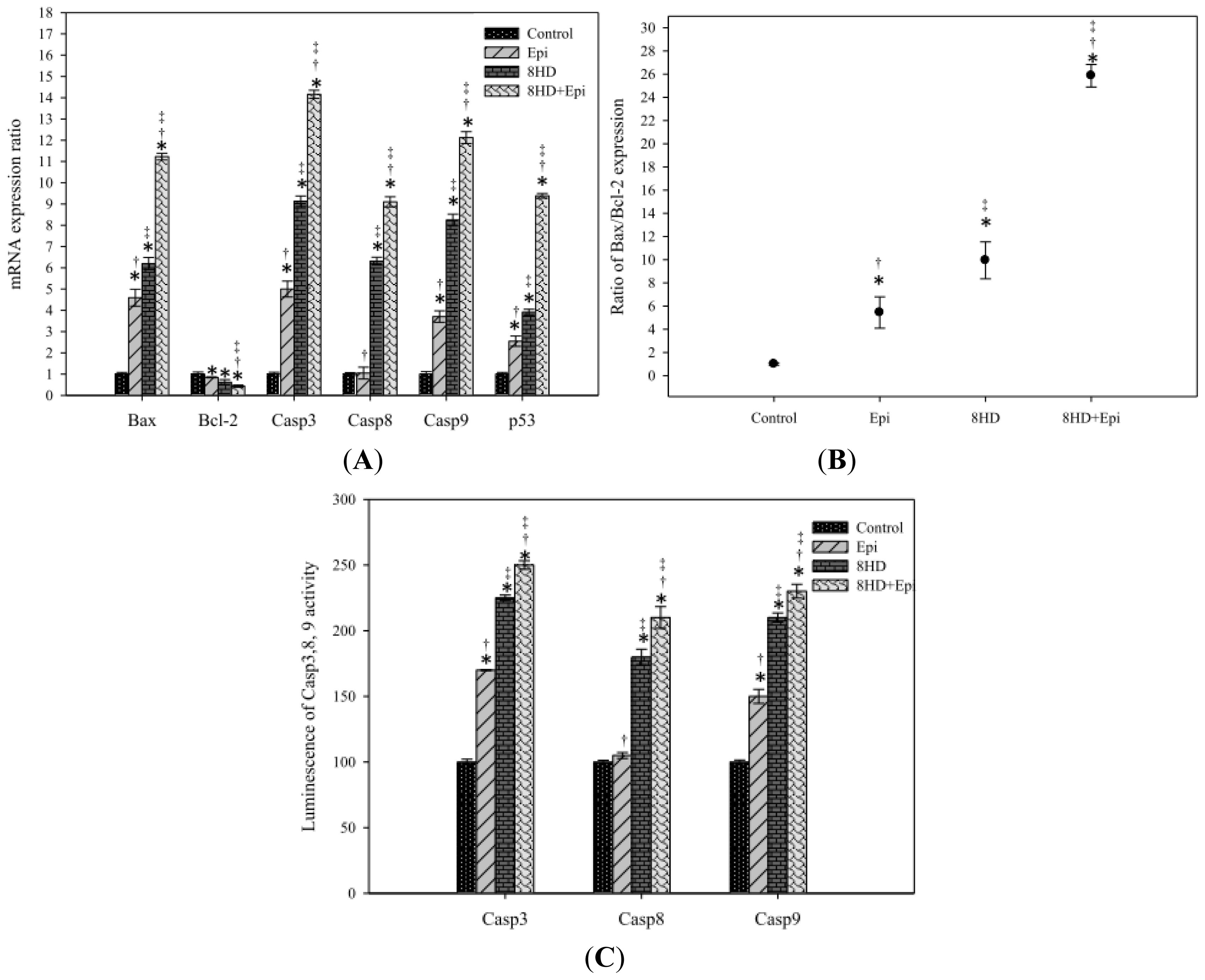
2.1.8. Combined 8HD with Epi-Modulated mRNA Expression Levels of Bax, Bcl-2, Caspases, and p53
2.1.9. Combined 8HD and Epi Treatment Increased Caspases-3, -8, and -9 Activities
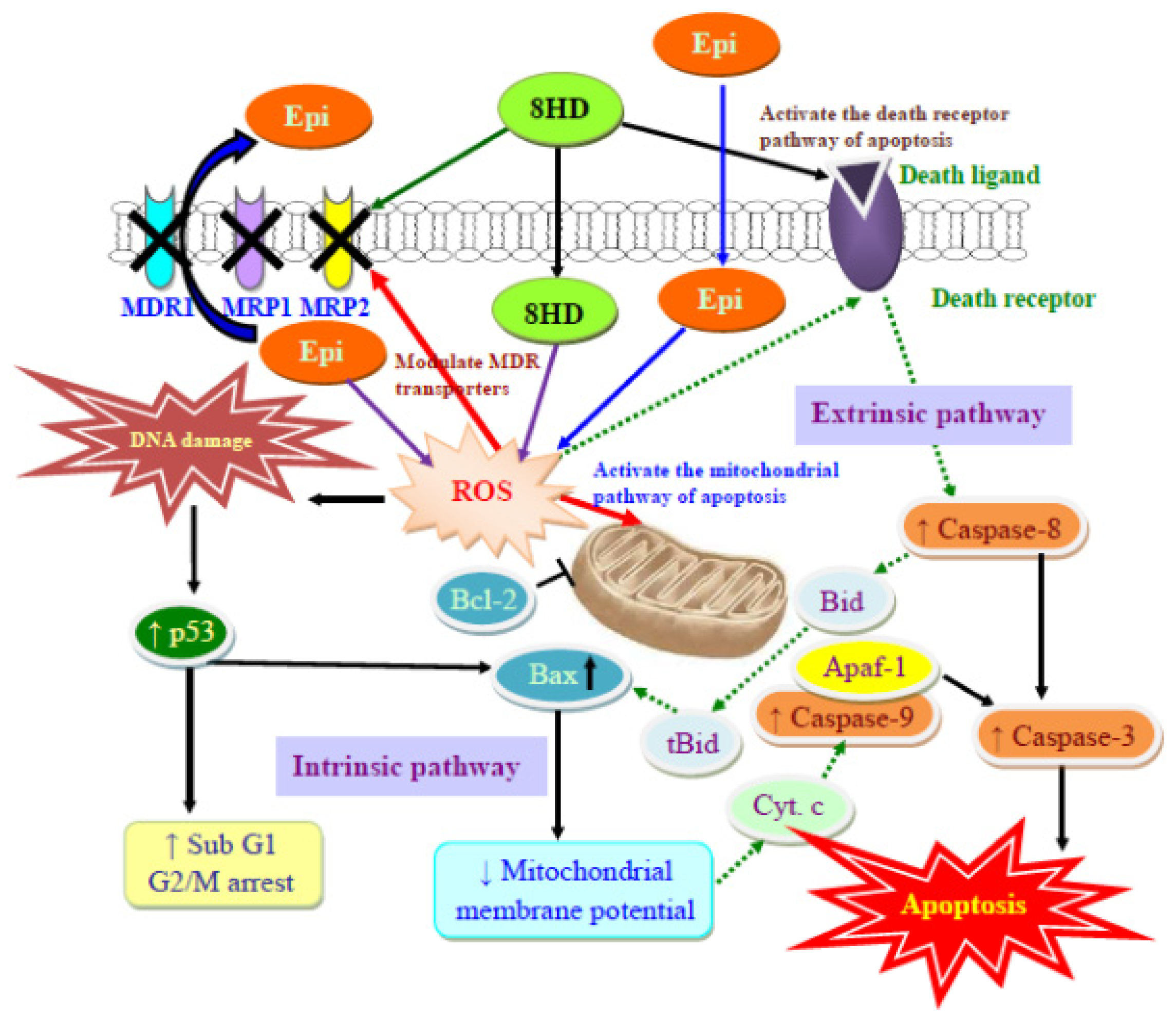
2.2. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Cell Growth Inhibition Assay and Isobologram Analysis
3.4. Determination of Intracellular Hydrogen Peroxide and Superoxide Oroduction
3.5. Real-Time PCR of P-gp, MRP1, MRP2, Bax, Bcl-2, Caspases, and p53
3.6. Measurement of Intracellular Epi Accumulation
3.7. Detection of Mitochondrial Membrane Potential
3.8. Determination of Cell Cycle
3.9. Detection of DNA Fragmentation
3.10. Caspases-3, -8, and -9 Activity Assay
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe author declares no conflict of interest.
References
- Guo, J.M.; Kang, G.Z.; Xiao, B.X.; Liu, D.H.; Zhang, S. Effect of daidzein on cell growth, cell cycle, and telomerase activity of human cervical cancer in vitro. Int. J. Gynecol. Cancer 2004, 14, 882–888. [Google Scholar]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin 2012, 62, 10–29. [Google Scholar]
- Liu, Y.Y.; Gupta, V.; Patwardhan, G.A.; Bhinge, K.; Zhao, Y.; Bao, J.; Mehendale, H.; Cabot, M.C.; Li, Y.T.; Jazwinski, S.M. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol. Cancer 2010, 9, 145. [Google Scholar]
- Wu, C.P.; Calcagno, A.M.; Ambudkar, S.V. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: Evaluation of current strategies. Curr. Mol. Pharmacol 2008, 1, 93–105. [Google Scholar]
- Lo, Y.L.; Ho, C.T.; Tsai, F.L. Inhibit multidrug resistance and induce apoptosis by using glycocholic acid and epirubicin. Eur. J. Pharm. Sci 2008, 35, 52–67. [Google Scholar]
- Wang, X.; Wang, C.; Qin, Y.W.; Yan, S.K.; Gao, Y.R. Simultaneous suppression of multidrug resistance and antiapoptotic cellular defense induces apoptosis in chemoresistant human acute myeloid leukemia cells. Leuk. Res 2007, 31, 989–994. [Google Scholar]
- Karwatsky, J.; Lincoln, M.C.; Georges, E. A mechanism for P-glycoprotein-mediated apoptosis as revealed by verapamil hypersensitivity. Biochemistry (Mosc) 2003, 42, 12163–12173. [Google Scholar]
- Pandey, V.; Chaube, B.; Bhat, M.K. Hyperglycemia regulates MDR-1, drug accumulation and ROS levels causing increased toxicity of carboplatin and 5-fluorouracil in MCF-7 cells. J. Cell. Biochem 2011, 112, 2942–2952. [Google Scholar]
- Wartenberg, M.; Richter, M.; Datchev, A.; Gunther, S.; Milosevic, N.; Bekhite, M.M.; Figulla, H.R.; Aran, J.M.; Petriz, J.; Sauer, H. Glycolytic pyruvate regulates P-glycoprotein expression in multicellular tumor spheroids via modulation of the intracellular redox state. J. Cell. Biochem 2010, 109, 434–446. [Google Scholar]
- Iwamaru, A.; Iwado, E.; Kondo, S.; Newman, R.A.; Vera, B.; Rodriguez, A.D.; Kondo, Y. Eupalmerin acetate, a novel anticancer agent from Caribbean gorgonian octocorals, induces apoptosis in malignant glioma cells via the c-Jun NH2-terminal kinase pathway. Mol. Cancer Ther 2007, 6, 184–192. [Google Scholar]
- Joshi, G.; Hardas, S.; Sultana, R.; St. Clair, D.K.; Vore, M.; Butterfield, D.A. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. J. Neurosci. Res 2007, 85, 497–503. [Google Scholar]
- Marbeuf-Gueye, C.; Salerno, M.; Quidu, P.; Garnier-Suillerot, A. Inhibition of the P-glycoprotein- and multidrug resistance protein-mediated efflux of anthracyclines and calceinacetoxymethyl ester by PAK-104P. Eur. J. Pharmacol 2000, 391, 207–216. [Google Scholar]
- Men, Y.; Wang, X.X.; Li, R.J.; Zhang, Y.; Tian, W.; Yao, H.J.; Ju, R.J.; Ying, X.; Zhou, J.; Li, N.; et al. The efficacy of mitochondrial targeting antiresistant epirubicin liposomes in treating resistant leukemia in animals. Int. J. Nanomed 2011, 6, 3125–3137. [Google Scholar]
- Kulling, S.E.; Honig, D.M.; Simat, T.J.; Metzler, M. Oxidative in vitro metabolism of the soy phytoestrogens daidzein and genistein. J. Agric. Food Chem 2000, 48, 4963–4972. [Google Scholar]
- Chang, T.S. Two potent suicide substrates of mushroom tyrosinase: 7,8,4′-trihydroxyisoflavone and 5,7,8,4′-tetrahydroxyisoflavone. J. Agric. Food Chem 2007, 55, 2010–2015. [Google Scholar]
- Chang, T.S.; Ding, H.Y.; Tai, S.S.K.; Wu, C.Y. Mushroom tyrosinase inhibitory effects of isoflavones isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288. Food Chem 2007, 105, 1430–1438. [Google Scholar]
- Tai, S.S.; Lin, C.G.; Wu, M.H.; Chang, T.S. Evaluation of depigmenting activity by 8-hydroxydaidzein in mouse B16 melanoma cells and human volunteers. Int. J. Mol. Sci 2009, 10, 4257–4266. [Google Scholar]
- Hirota, A.; Inaba, M.; Chen, Y.C.; Abe, N.; Taki, S.; Yano, M.; Kawaii, S. Isolation of 8-hydroxyglycitein and 6-hydroxydaidzein from soybean miso. Biosci. Biotechnol. Biochem 2004, 68, 1372–1374. [Google Scholar]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci 2009, 10, 2440–2475. [Google Scholar]
- Hedlund, T.E.; van Bokhoven, A.; Johannes, W.U.; Nordeen, S.K.; Ogden, L.G. Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. Prostate 2006, 66, 557–566. [Google Scholar]
- Chang, T.L. Inhibitory effect of flavonoids on 26S proteasome activity. J. Agric. Food Chem 2009, 57, 9706–9715. [Google Scholar]
- Park, J.S.; Kim, D.H.; Lee, J.K.; Lee, J.Y.; Kim, H.K.; Lee, H.J.; Kim, H.C. Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorg. Med. Chem. Lett 2010, 20, 1162–1164. [Google Scholar]
- Hirota, A.; Taki, S.; Kawaii, S.; Yano, M.; Abe, N. 1,1-Diphenyl-2-picrylhydrazyl radical-scavenging compounds from soybean miso and antiproliferative activity of isoflavones from soybean miso toward the cancer cell lines. Biosci. Biotechnol. Biochem 2000, 64, 1038–1040. [Google Scholar]
- Chen, J.; Lin, H.; Hu, M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother. Pharmacol 2005, 55, 159–169. [Google Scholar]
- Esaki, H.; Shirasaki, T.; Yamashita, K.; Nakamura, Y.; Kawakishi, S.; Osawa, T. Absorption and excretion of the 8-hydroxydaidzein in rats after oral administration and its antioxidant effect. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 80–86. [Google Scholar]
- Enokizono, J.; Kusuhara, H.; Ose, A.; Schinkel, A.H.; Sugiyama, Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab. Dispos 2008, 36, 995–1002. [Google Scholar]
- Janneh, O.; Anwar, T.; Jungbauer, C.; Kopp, S.; Khoo, S.H.; Back, D.J.; Chiba, P. P-glycoprotein, multidrug resistance-associated proteins and human organic anion transporting polypeptide influence the intracellular accumulation of atazanavir. Antiviral Ther 2009, 14, 965–974. [Google Scholar]
- Choi, E.J. The prooxidant, rather than antioxidant, acts of daidzein in vivo and in vitro: Daidzein suppresses glutathione metabolism. Eur. J. Pharmacol 2006, 542, 162–169. [Google Scholar]
- Rabiau, N.; Kossai, M.; Braud, M.; Chalabi, N.; Satih, S.; Bignon, Y.J.; Bernard-Gallon, D.J. Genistein and daidzein act on a panel of genes implicated in cell cycle and angiogenesis by polymerase chain reaction arrays in human prostate cancer cell lines. Cancer Epidemiol 2010, 34, 200–206. [Google Scholar]
- Totta, P.; Acconcia, F.; Virgili, F.; Cassidy, A.; Weinberg, P.D.; Rimbach, G.; Marino, M. Daidzein-sulfate metabolites affect transcriptional and antiproliferative activities of estrogen receptor-beta in cultured human cancer cells. J. Nutr 2005, 135, 2687–2693. [Google Scholar]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol 2010, 21, 263–268. [Google Scholar]
- Choi, E.J.; Kim, G.H. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine 2008, 15, 683–690. [Google Scholar]
- Gutmann, H.; Fricker, G.; Torok, M.; Michael, S.; Beglinger, C.; Drewe, J. Evidence for different ABC-transporters in Caco-2 cells modulating drug uptake. Pharm. Res 1999, 16, 402–407. [Google Scholar]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J. Chemother 2005, 17, 86–95. [Google Scholar]
- Wartenberg, M.; Ling, F.C.; Schallenberg, M.; Baumer, A.T.; Petrat, K.; Hescheler, J.; Sauer, H. Down-regulation of intrinsic P-glycoprotein expression in multicellular prostate tumor spheroids by reactive oxygen species. J. Biol. Chem 2001, 276, 17420–17428. [Google Scholar]
- Wartenberg, M.; Gronczynska, S.; Bekhite, M.M.; Saric, T.; Niedermeier, W.; Hescheler, J.; Sauer, H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular prostate tumor spheroids by hyperthermia and reactive oxygen species. Int. J. Cancer 2005, 113, 229–240. [Google Scholar]
- Wu, C.P.; Calcagno, A.M.; Hladky, S.B.; Ambudkar, S.V.; Barrand, M.A. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J 2005, 272, 4725–4740. [Google Scholar]
- Cai, Y.; Lu, J.; Miao, Z.; Lin, L.; Ding, J. Reactive oxygen species contribute to cell killing and P-glycoprotein downregulation by salvicine in multidrug resistant K562/A02 cells. Cancer Biol. Ther 2007, 6, 1794–1799. [Google Scholar]
- Wang, H.Z.; Zhang, Y.; Xie, L.P.; Yu, X.Y.; Zhang, R.Q. Effects of genistein and daidzein on the cell growth, cell cycle, and differentiation of human and murine melanoma cells. J. Nutr. Biochem 2002, 13, 421–426. [Google Scholar]
- Yang, J.; Wu, L.J.; Tashino, S.; Onodera, S.; Ikejima, T. Protein tyrosine kinase pathway-derived ROS/NO productions contribute to G2/M cell cycle arrest in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic. Res 2010, 44, 792–802. [Google Scholar]
- Lo, F.H.; Mak, N.K.; Leung, K.N. Studies on the anti-tumor activities of the soy isoflavone daidzein on murine neuroblastoma cells. Biomed. Pharmacother 2007, 61, 591–595. [Google Scholar]
- Shabbits, J.A.; Hu, Y.; Mayer, L.D. Tumor chemosensitization strategies based on apoptosis manipulations. Mol. Cancer Ther 2003, 2, 805–813. [Google Scholar]
- Wang, B.F.; Wang, J.S.; Lu, J.F.; Kao, T.H.; Chen, B.H. Antiproliferation effect and mechanism of prostate cancer cell lines as affected by isoflavones from soybean cake. J. Agric. Food Chem 2009, 57, 2221–2232. [Google Scholar]
- Hartwell, L.H.; Kastan, M.B. Cell cycle control and cancer. Science 1994, 266, 1821–1828. [Google Scholar]
- Inoue, H.; Waiwut, P.; Saiki, I.; Shimada, Y.; Sakurai, H. Gomisin N enhances TRAIL-induced apoptosis via reactive oxygen species-mediated up-regulation of death receptors 4 and 5. Int. J. Oncol 2012, 40, 1058–1065. [Google Scholar]
- Tallarida, R.J. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther 2006, 319, 1–7. [Google Scholar]
- Tong, N.; Zhang, J.; Chen, Y.; Li, Z.; Luo, Y.; Zuo, H.; Zhao, X. Berberine sensitizes mutliple human cancer cells to the anticancer effects of doxorubicin in vitro. Oncol. Lett 2012, 3, 1263–1267. [Google Scholar]
- Liu, C.; Wang, Y.; Xie, S.; Zhou, Y.; Ren, X.; Li, X.; Cai, Y. Liquiritigenin induces mitochondria-mediated apoptosis via cytochrome c release and caspases activation in HeLa Cells. Phytother. Res 2011, 25, 277–283. [Google Scholar]

| Primer name | Forward primer sequences | Reverse primer sequences |
|---|---|---|
| GAPDH | ATGGGGAAGGTGAAGGTCG | GGGGTCATTGATGGCAACAATA |
| MDR1 | GCTCATCGTTTGTCTACAGTTCGT | ACAATGACTCCATCATCGAAACC |
| MRP1 | GGATCATGCTCACTTTCTGG | AAGTGATGTCACGAAACAGGTC |
| MRP2 | AAGATGCAGCCTCCATAACCA | TGGACCTAGAACTGCGGCTAA |
| p53 | GAGAATCTCCGCAAGAAAGG | CTCATTCAGCTCTCGGAACA |
| Bcl-2 | CTTGACAGAGGATCATGCTGTAC | GGATGCTTTATTTCATGAGGC |
| Bax | GGGCCCACCAGCTCTGA | CCTGCTCGATCCTGGATGA |
| Caspase-3 | CCTGGTTATTATTCTTGGCGAAA | GCACAAAGCGACTGGATGAA |
| Caspase-8 | CAGGCAGGGCTCAAATTTCT | TCTGCTCACTTCTTCTGAAATCTGA |
| Caspase-9 | TGCTGAGCAGCGAGCTGTT | AGCCTGCCCGCTGGAT |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lo, Y.-L. A Potential Daidzein Derivative Enhances Cytotoxicity of Epirubicin on Human Colon Adenocarcinoma Caco-2 Cells. Int. J. Mol. Sci. 2013, 14, 158-176. https://doi.org/10.3390/ijms14010158
Lo Y-L. A Potential Daidzein Derivative Enhances Cytotoxicity of Epirubicin on Human Colon Adenocarcinoma Caco-2 Cells. International Journal of Molecular Sciences. 2013; 14(1):158-176. https://doi.org/10.3390/ijms14010158
Chicago/Turabian StyleLo, Yu-Li. 2013. "A Potential Daidzein Derivative Enhances Cytotoxicity of Epirubicin on Human Colon Adenocarcinoma Caco-2 Cells" International Journal of Molecular Sciences 14, no. 1: 158-176. https://doi.org/10.3390/ijms14010158




