Changes in Translational Control after Pro-Apoptotic Stress
Abstract
:1. Introduction
2. Inhibition of Global Protein Synthesis Due to Inactivation of Translation Initiation Factors
2.1. eIF2 and eIF2B
2.2. eIF3 and eIF4F
3. Selective Translation of mRNAs Encoding Pro-Survival or Pro-Apoptotic Proteins
3.1. uORF-Dependent Translation
3.2. IRES-Dependent Translation
4. Conclusions
Acknowledgments
- Conflict of interestThe authors declare no conflict of interest.
References
- Dever, T.E. Translation initiation: Adept at adapting. Trends Biochem. Sci 1999, 24, 398–403. [Google Scholar]
- Schmitt, E.; Naveau, M.; Mechulam, Y. Eukaryotic and archaeal translation initiation factor 2: A heterotrimeric tRNA carrier. FEBS Lett 2010, 584, 405–412. [Google Scholar]
- Levin, D.; London, I.M. Regulation of protein synthesis: Activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc. Natl. Acad. Sci. USA 1978, 75, 1121–1125. [Google Scholar]
- García, M.A.; Meurs, E.F.; Esteban, M. The dsRNA protein kinase PKR: Virus and cell control. Biochimie 2007, 89, 799–811. [Google Scholar]
- Lee, S.B.; Esteban, M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology 1994, 199, 491–496. [Google Scholar]
- Yeung, M.C.; Lau, A.S. Tumor suppressor p53 as a component of the tumor necrosis factor-induced, protein kinase PKR-mediated apoptotic pathway in human promonocytic U937 cells. J. Biol. Chem 1998, 273, 25198–25202. [Google Scholar]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar]
- Wek, R.C.; Jiang, H.Y.; Anthony, T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans 2006, 34, 7–11. [Google Scholar]
- Berlanga, J.J.; Santoyo, J.; de Haro, C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2α kinase. Eur. J. Biochem 1999, 265, 754–762. [Google Scholar]
- Hinnebusch, A.G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc. Natl. Acad. Sci. USA 1984, 81, 6442–6446. [Google Scholar]
- Ye, J.; Kumanova, M.; Hart, L.S.; Sloane, K.; Zhang, H.; de Panis, D.N.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Ron, D.; Koumenis, C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J 2010, 29, 2082–2096. [Google Scholar]
- Liu, Y.; László, C.; Liu, Y.; Liu, W.; Chen, X.; Evans, S.C.; Wu, S. Regulation of G(1) arrest and apoptosis in hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation. Neoplasia 2010, 12, 61–68. [Google Scholar]
- Jiang, H.Y.; Wek, R.C. GCN2 phosphorylation of eIF2α activates NF-κB in response to UV irradiation. Biochem. J 2005, 385, 371–380. [Google Scholar]
- Marissen, W.E.; Guo, Y.; Thomas, A.A.; Matts, R.L.; Lloyd, R.E. Identification of caspase 3-mediated cleavage and functional alteration of eukaryotic initiation factor 2alpha in apoptosis. J. Biol. Chem 2000, 275, 9314–9323. [Google Scholar]
- Satoh, S.; Hijikata, M.; Handa, H.; Shimotohno, K. Caspase-mediated cleavage of eukaryotic translation initiation factor subunit 2alpha. Biochem. J 1999, 342, 65–70. [Google Scholar]
- Welsh, G.I.; Miller, C.M.; Loughlin, A.J.; Price, N.T.; Proud, C.G. Regulation of eukaryotic initiation factor eIF2B: Glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett 1998, 421, 125–130. [Google Scholar]
- Wang, X.; Proud, C.G. A novel mechanism for the control of translation initiation by amino acids, mediated by phosphorylation of eukaryotic initiation factor 2B. Mol. Cell. Biol 2008, 28, 1429–1442. [Google Scholar]
- Woo, C.W.; Kutzler, L.; Kimball, S.R.; Tabas, I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat. Cell Biol 2012, 14, 192–200. [Google Scholar]
- Hui, D.J.; Bhasker, C.R.; Merrick, W.C.; Sen, G.C. Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2·GTP·Met-tRNAi. J. Biol. Chem 2003, 278, 39477–39482. [Google Scholar]
- Fensterl, V.; Sen, G.C. The ISG56/IFIT1 gene family. J. Interferon Cytokine Res 2011, 31, 71–78. [Google Scholar]
- Zhang, L.; Pan, X.; Hershey, J.W. Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J. Biol. Chem 2007, 282, 5790–5800. [Google Scholar]
- Bushell, M.; Wood, W.; Clemens, M.J.; Morley, S.J. Changes in integrity and association of eukaryotic protein synthesis initiation factors during apoptosis. Eur. J. Biochem 2000, 267, 1083–1091. [Google Scholar]
- Shi, J.; Feng, Y.; Goulet, A.C.; Vaillancourt, R.R.; Sachs, N.A.; Hershey, J.W.; Nelson, M.A. The p34cdc2-related cyclin-dependent kinase 11 interacts with the p47 subunit of eukaryotic initiation factor 3 during apoptosis. J. Biol. Chem 2003, 278, 5062–5071. [Google Scholar]
- Lin, Y.M.; Chen, Y.R.; Lin, J.R.; Wang, W.J.; Inoko, A.; Inagaki, M.; Wu, Y.C.; Chen, R.H. eIF3k regulates apoptosis in epithelial cells by releasing caspase 3 from keratin-containing inclusions. J. Cell Sci 2008, 121, 2382–2393. [Google Scholar]
- Martineau, Y.; Azar, R.; Bousquet, C.; Pyronnet, S. Anti-oncogenic potential of the eIF4E-binding proteins. Oncogene 2012. [Google Scholar] [CrossRef]
- Pyronnet, S.; Imataka, H.; Gingras, A.C.; Fukunaga, R.; Hunter, T.; Sonenberg, N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J 1999, 18, 270–279. [Google Scholar]
- Pyronnet, S. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochem. Pharmacol 2000, 60, 1237–1243. [Google Scholar]
- Holcik, M.; Sonenberg, N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol 2005, 6, 318–327. [Google Scholar]
- Tee, A.R.; Proud, C.G. Caspase cleavage of initiation factor 4E-binding protein 1 yields a dominant inhibitor of cap-dependent translation and reveals a novel regulatory motif. Mol. Cell. Biol. 2002, 22, 1674–1683. [Google Scholar]
- Li, Q.; Imataka, H.; Morino, S.; Rogers, G.W., Jr; Richter-Cook, N.J.; Merrick, W.C.; Sonenberg, N. Eukaryotic translation initiation factor 4AIII (eIF4AIII) is functionally distinct from eIF4AI and eIF4AII. Mol. Cell. Biol. 1999, 19, 7336–7346. [Google Scholar]
- Yang, H.S.; Jansen, A.P.; Nair, R.; Shibahara, K.; Verma, A.K.; Cmarik, J.L.; Colburn, N.H. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene 2001, 20, 669–676. [Google Scholar]
- Yang, H.S.; Jansen, A.P.; Komar, A.A.; Zheng, X.; Merrick, W.C.; Costes, S.; Lockett, S.J.; Sonenberg, N.; Colburn, N.H. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol. Cell. Biol 2003, 23, 26–37. [Google Scholar]
- Gradi, A.; Imataka, H.; Svitkin, Y.V.; Rom, E.; Raught, B.; Morino, S.; Sonenberg, N. A novel functional human eukaryotic translation initiation factor 4G. Mol. Cell. Biol 1998, 18, 334–342. [Google Scholar]
- Ling, J.; Morley, S.J.; Traugh, J.A. Inhibition of cap-dependent translation via phosphorylation of eIF4G by protein kinase Pak2. EMBO J 2005, 24, 4094–4105. [Google Scholar]
- Henis-Korenblit, S.; Shani, G.; Sines, T.; Marash, L.; Shohat, G.; Kimchi, A. The caspase-cleaved DAP5 protein supports internal ribosome entry site-mediated translation of death proteins. Proc. Natl. Acad. Sci. USA 2002, 99, 5400–5405. [Google Scholar]
- Marissen, W.E.; Gradi, A.; Sonenberg, N.; Lloyd, R.E. Cleavage of eukaryotic translation initiation factor 4GII correlates with translation inhibition during apoptosis. Cell Death Differ 2000, 7, 1234–1243. [Google Scholar]
- Imataka, H.; Gradi, A.; Sonenberg, N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J 1998, 17, 7480–7489. [Google Scholar]
- Marissen, W.E.; Triyoso, D.; Younan, P.; Lloyd, R.E. Degradation of poly(A)-binding protein in apoptotic cells and linkage to translation regulation. Apoptosis 2004, 9, 67–75. [Google Scholar]
- Imataka, H.; Olsen, H.S.; Sonenberg, N. A new translational regulator with homology to eukaryotic translation initiation factor 4G. EMBO J 1997, 16, 817–825. [Google Scholar]
- Lee, S.H.; McCormick, F. p97/DAP5 is a ribosome-associated factor that facilitates protein synthesis and cell proliferation by modulating the synthesis of cell cycle proteins. EMBO J 2006, 25, 4008–4019. [Google Scholar]
- Yamanaka, S.; Zhang, X.Y.; Maeda, M.; Miura, K.; Wang, S.; Farese, R.V., Jr; Iwao, H.; Innerarity, T.L. Essential role of NAT1/p97/DAP5 in embryonic differentiation and the retinoic acid pathway. EMBO J. 2000, 19, 5533–5541. [Google Scholar]
- Henis-Korenblit, S.; Strumpf, N.L.; Goldstaub, D.; Kimchi, A. A novel form of DAP5 protein accumulates in apoptotic cells as a result of caspase cleavage and internal ribosome entry site-mediated translation. Mol. Cell. Biol 2000, 20, 496–506. [Google Scholar]
- Lu, P.D.; Harding, H.P.; Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol 2004, 167, 27–33. [Google Scholar]
- Vattem, K.M.; Wek, R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11269–11274. [Google Scholar]
- Palam, L.R.; Baird, T.D.; Wek, R.C. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem 2011, 286, 10939–10949. [Google Scholar]
- Lee, Y.Y.; Cevallos, R.C.; Jan, E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2α phosphorylation. J. Biol. Chem 2009, 284, 6661–6673. [Google Scholar]
- Fawcett, T.W.; Martindale, J.L.; Guyton, K.Z.; Hai, T.; Holbrook, N.J. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem. J 1999, 339, 135–141. [Google Scholar]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol 2001, 21, 1249–1259. [Google Scholar]
- Yamaguchi, H.; Wang, H.G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem 2004, 279, 45495–45502. [Google Scholar]
- Ohoka, N.; Yoshii, S.; Hattori, T.; Onozaki, K.; Hayashi, H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 2005, 24, 1243–1255. [Google Scholar]
- Warnakulasuriyarachchi, D.; Ungureanu, N.H.; Holcík, M. The translation of an antiapoptotic protein HIAP2 is regulated by an upstream open reading frame. Cell Death Differ 2003, 10, 899–904. [Google Scholar]
- Warnakulasuriyarachchi, D.; Cerquozzi, S.; Cheung, H.H.; Holcík, M. Translational induction of the inhibitor of apoptosis protein HIAP2 during endoplasmic reticulum stress attenuates cell death and is mediated via an inducible internal ribosome entry site element. J. Biol. Chem 2004, 279, 17148–17157. [Google Scholar]
- Holcik, M.; Korneluk, R.G. Functional characterization of the X-linked inhibitor of apoptosis (XIAP) internal ribosome entry site element: Role of La autoantigen in XIAP translation. Mol. Cell. Biol 2000, 20, 4648–4657. [Google Scholar]
- Li, X.; Gong, R.Y.; Wang, M.; Yan, Z.L.; Yuan, B.; Wang, K.; Shi, L.H. Sorafenib down-regulates c-IAP expression post-transcriptionally in hepatic carcinoma cells to suppress apoptosis. Biochem. Biophys. Res. Commun 2012, 418, 531–536. [Google Scholar]
- Sherrill, K.W.; Byrd, M.P.; van Eden, M.E.; Lloyd, R.E. BCL-2 translation is mediated via internal ribosome entry during cell stress. J. Biol. Chem 2004, 279, 29066–29074. [Google Scholar]
- Yoon, A.; Peng, G.; Brandenburger, Y.; Zollo, O.; Xu, W.; Rego, E.; Ruggero, D. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science 2006, 312, 902–906. [Google Scholar]
- Coldwell, M.J.; Mitchell, S.A.; Stoneley, M.; MacFarlane, M.; Willis, A.E. Initiation of Apaf-1 translation by internal ribosome entry. Oncogene 2000, 19, 899–905. [Google Scholar]
- Nanbru, C.; Lafon, I.; Audigier, S.; Gensac, M.C.; Vagner, S.; Huez, G.; Prats, A.C. Alternative translation of the proto-oncogene c-myc by an internal ribosome entry site. J. Biol. Chem 1997, 272, 32061–32066. [Google Scholar]
- Yeh, S.H.; Yang, W.B.; Gean, P.W.; Hsu, C.Y.; Tseng, J.T.; Su, T.P.; Chang, W.C.; Hung, J.J. Translational and transcriptional control of Sp1 against ischaemia through a hydrogen peroxide-activated internal ribosomal entry site pathway. Nucl. Acids Res 2011, 39, 5412–5423. [Google Scholar]
- Marash, L.; Liberman, N.; Henis-Korenblit, S.; Sivan, G.; Reem, E.; Elroy-Stein, O.; Kimchi, A. DAP5 promotes cap-independent translation of Bcl-2 and CDK1 to facilitate cell survival during mitosis. Mol. Cell 2008, 30, 447–459. [Google Scholar]
- Macejak, D.G.; Sarnow, P. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature 1991, 353, 90–94. [Google Scholar]
- Yaman, I.; Fernandez, J.; Liu, H.; Caprara, M.; Komar, A.A.; Koromilas, A.E.; Zhou, L.; Snider, M.D.; Scheuner, D.; Kaufman, R.J.; et al. The zipper model of translational control: A small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell 2003, 113, 519–531. [Google Scholar]
- Morrish, B.C.; Rumsby, M.G. The 5′ untranslated region of protein kinase Cdelta directs translation by an internal ribosome entry segment that is most active in densely growing cells and during apoptosis. Mol. Cell. Biol 2002, 22, 6089–6099. [Google Scholar]
- Bushell, M.; Stoneley, M.; Kong, Y.W.; Hamilton, T.L.; Spriggs, K.A.; Dobbyn, H.C.; Qin, X.; Sarnow, P.; Willis, A.E. Polypyrimidine tract binding protein regulates IRES-mediated gene expression during apoptosis. Mol. Cell 2006, 23, 401–412. [Google Scholar]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar]
- Mott, J.L.; Kobayashi, S.; Bronk, S.F.; Gores, G.J. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007, 26, 6133–6140. [Google Scholar]
- Shimizu, S.; Takehara, T.; Hikita, H.; Kodama, T.; Miyagi, T.; Hosui, A.; Tatsumi, T.; Ishida, H.; Noda, T.; Nagano, H.; et al. The let-7 family of microRNAs inhibits Bcl-xL expression and potentiates sorafenib-induced apoptosis in human hepatocellular carcinoma. J. Hepatol 2010, 52, 698–704. [Google Scholar]
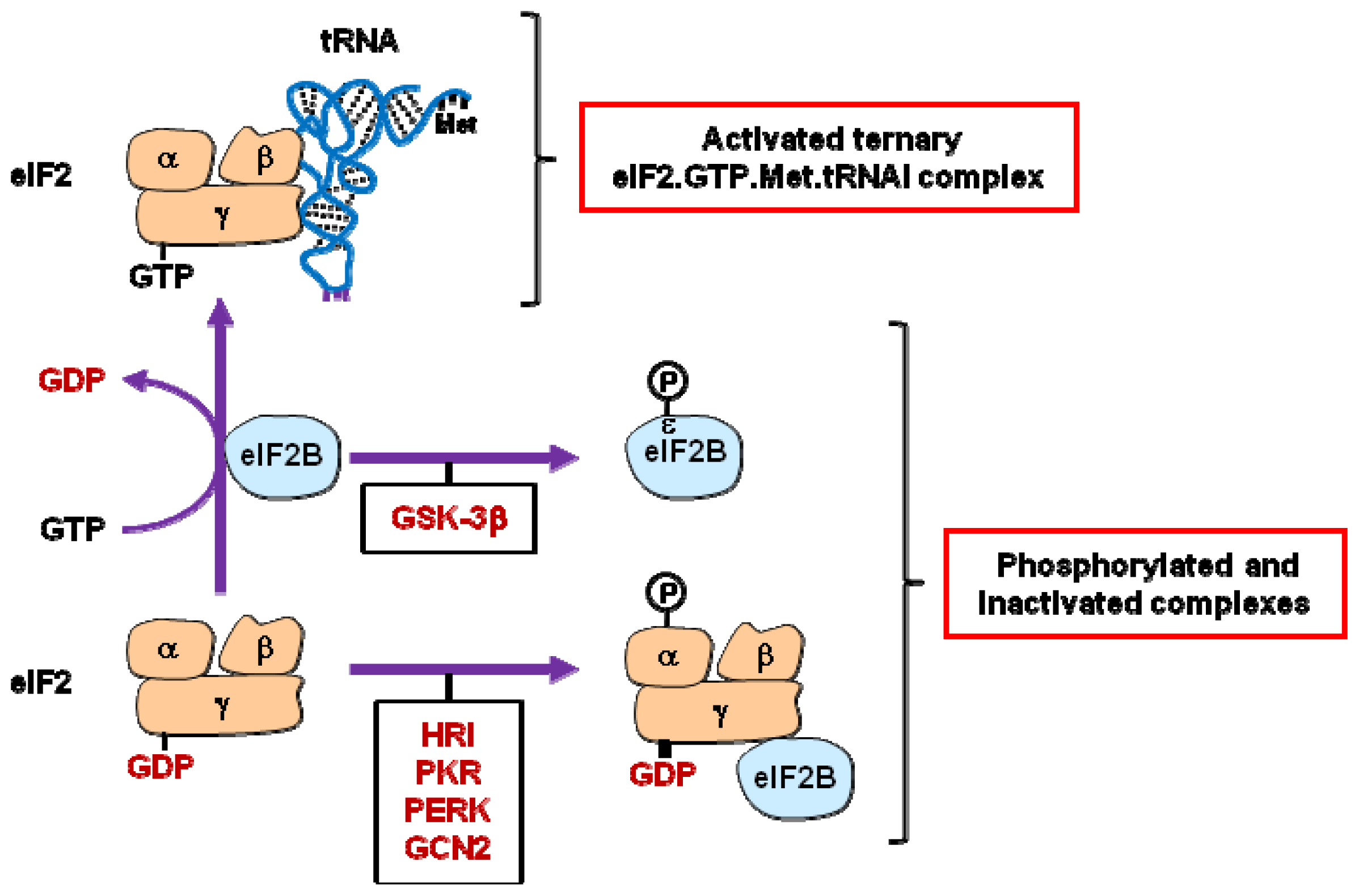
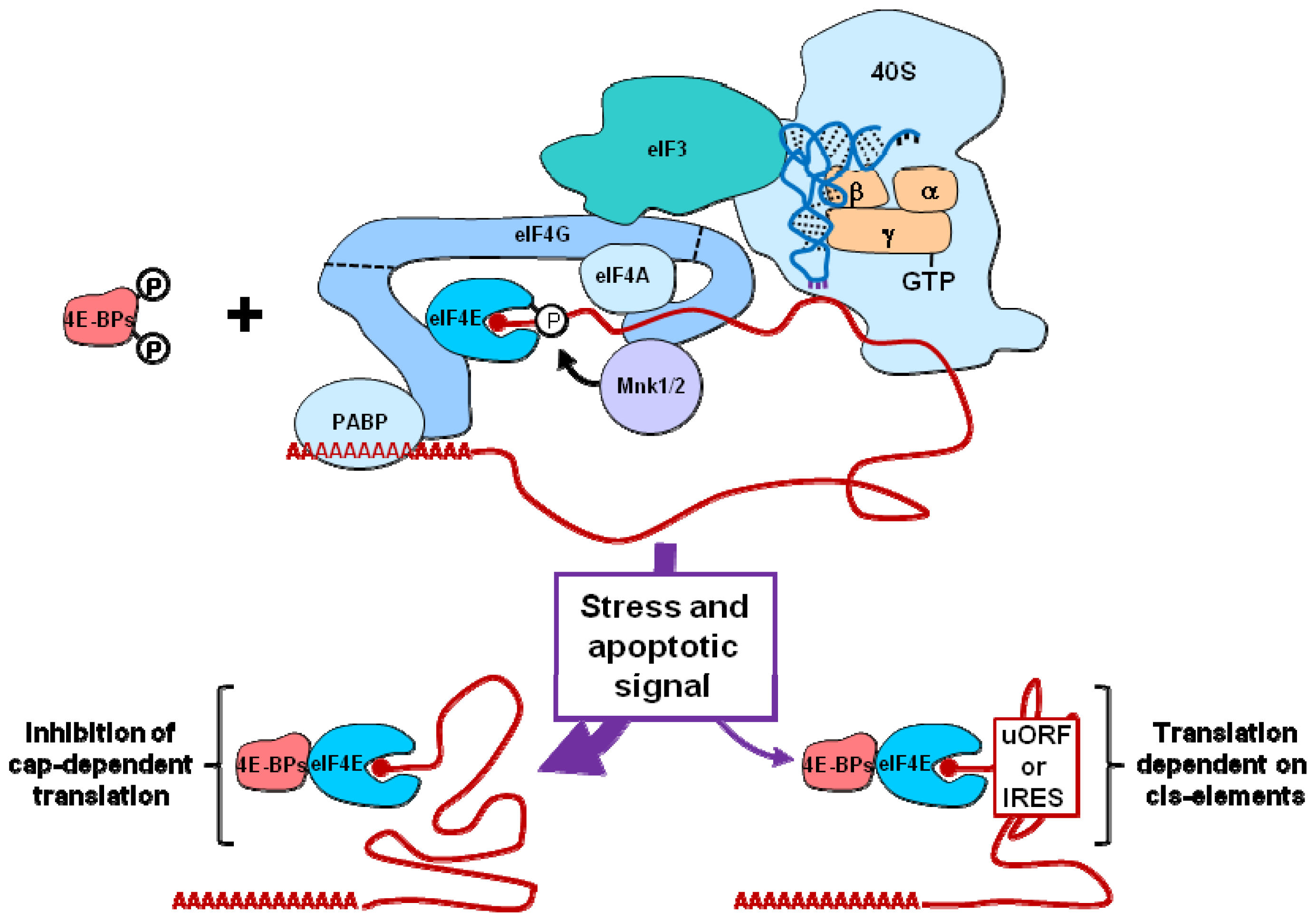
| Translation initiation factor | Modifications of initiation factors involved in inhibition of cap-dependent translation | Involvement of modified initiation factors in selective translation dependent on cis-elements |
|---|---|---|
| eIF2 | .Phosphorylation of eIF2α [5,8–10] | .Relief of uORF-mediated translation initiation [44–47] |
| .Caspase cleavage of eIF2α [15,16] | ||
| eIF2B | .Phosphorylation of eIF2Bɛ [17–19] | |
| eIF3 | .Caspase cleavage of eIF3j [23] | |
| .Phosphorylation of eIF3f by CDK11 [24] | ||
| .Interaction of eIF3c or eIF3e with ISG56/IFIT1 [21] | ||
| .Sequestration by eIF4G caspase cleavage fragments [36] | ||
| 4E-BP1/2 | .Caspase cleavage of 4E-BP1 [30] | |
| eIF4F | ||
| eIF4E | .Sequestration by hypophosphorylated and cleaved 4E-BP1/2 [29,30] | |
| .Sequestration by eIF4GI/II-caspase-cleavage fragments [36] | ||
| eIF4AI/II | .Interaction with PDCD4 [33] | |
| .Sequestration by eIF4GI/II-caspase-cleavage fragments [36] | ||
| eIF4GI/II | .Phosphorylation of eIF4GI by PAK2 [35] | |
| .Caspase cleavage of eIF4GI and eIF4GII [36] | ||
| PABP | .Caspase cleavage [39] | |
| .Sequestration by eIF4GI/II-caspase-cleavage fragments [36] | ||
| P97/DAP5 | .Induction of IRES-mediated translation [53–55] | |
| uORF | IRES | ||||
|---|---|---|---|---|---|
| Function | Survival | Death | Survival | Death | References |
| Transcription | ATF4 | [45] | |||
| CHOP | [46] | ||||
| c-Myc | [59] | ||||
| Sp1 | [60] | ||||
| IAP family (survival) | HIAP | [53] | |||
| XIAP | [54] | ||||
| c-IAP | [55] | ||||
| Bcl family (survival) | Bcl-2 | [56] | |||
| Bcl-x(L) | [57] | ||||
| Apotosome | APAF-1 | [58] | |||
| Cell cycle | CDK1 | [61] | |||
| Chaperone | Bip | [62] | |||
| Translation | P97/DAP5 | P97/DAP5 | [43] | ||
| Signaling | GADD34 | [47] | |||
| PKCδ | [64] | ||||
| Amino-acid transport | Cat-1 | Cat-1 | [63] | ||
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lasfargues, C.; Martineau, Y.; Bousquet, C.; Pyronnet, S. Changes in Translational Control after Pro-Apoptotic Stress. Int. J. Mol. Sci. 2013, 14, 177-190. https://doi.org/10.3390/ijms14010177
Lasfargues C, Martineau Y, Bousquet C, Pyronnet S. Changes in Translational Control after Pro-Apoptotic Stress. International Journal of Molecular Sciences. 2013; 14(1):177-190. https://doi.org/10.3390/ijms14010177
Chicago/Turabian StyleLasfargues, Charline, Yvan Martineau, Corinne Bousquet, and Stéphane Pyronnet. 2013. "Changes in Translational Control after Pro-Apoptotic Stress" International Journal of Molecular Sciences 14, no. 1: 177-190. https://doi.org/10.3390/ijms14010177




