Pre-Treatment of Platinum Resistant Ovarian Cancer Cells with an MMP-9/MMP-2 Inhibitor Prior to Cisplatin Enhances Cytotoxicity as Determined by High Content Screening
Abstract
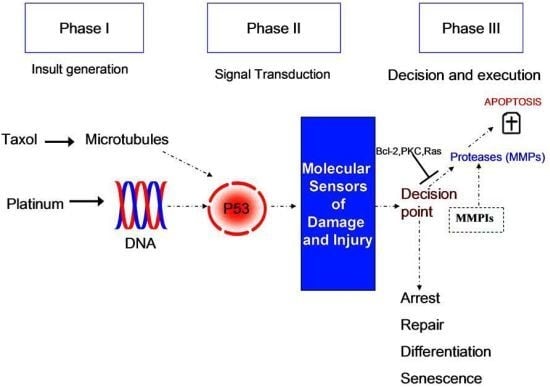
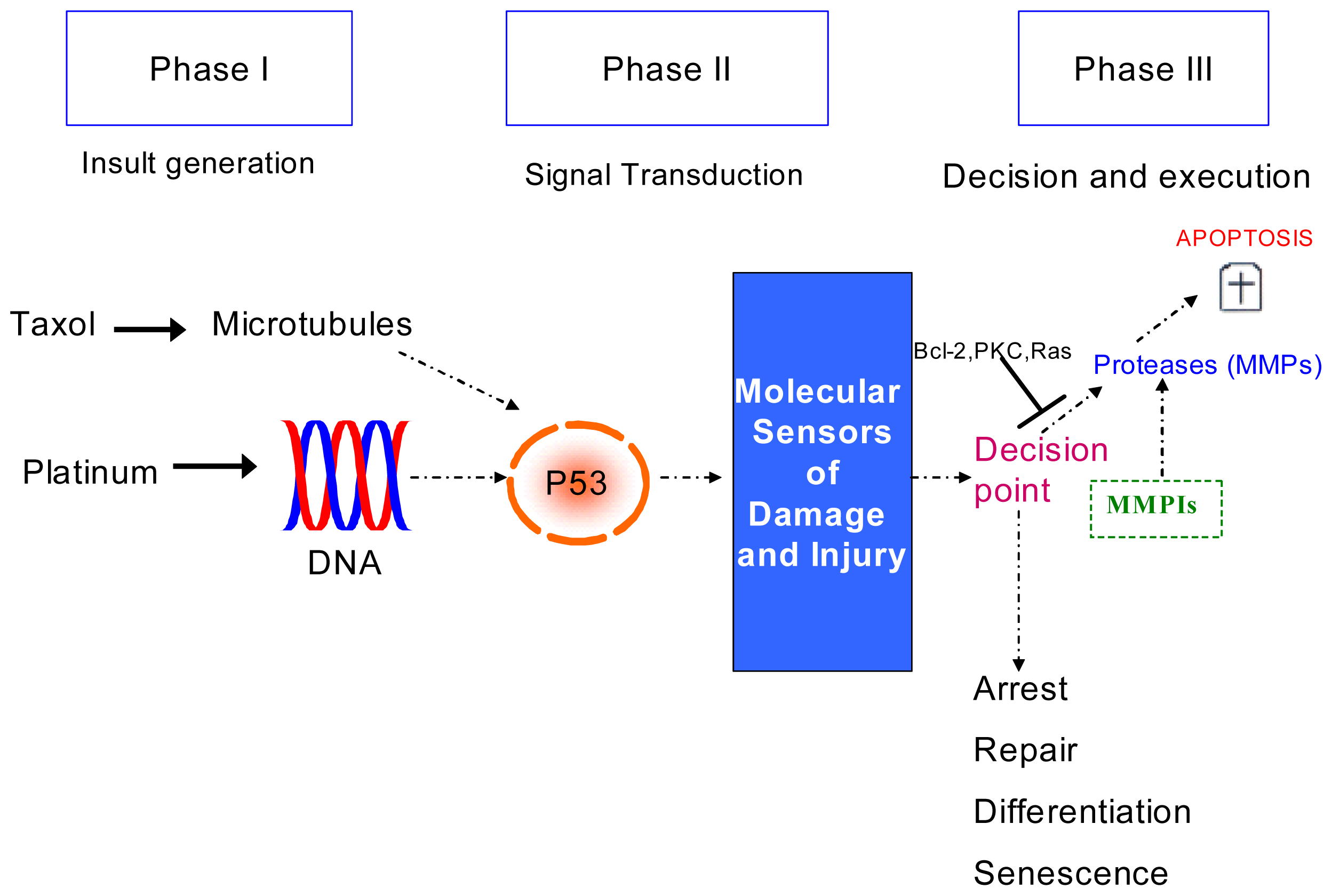
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. RNA Sample Analysis from Cell Lines
2.1.2. Cisplatin Induces Cell Death in Cisplatin Resistant Ovarian Cancer Cells
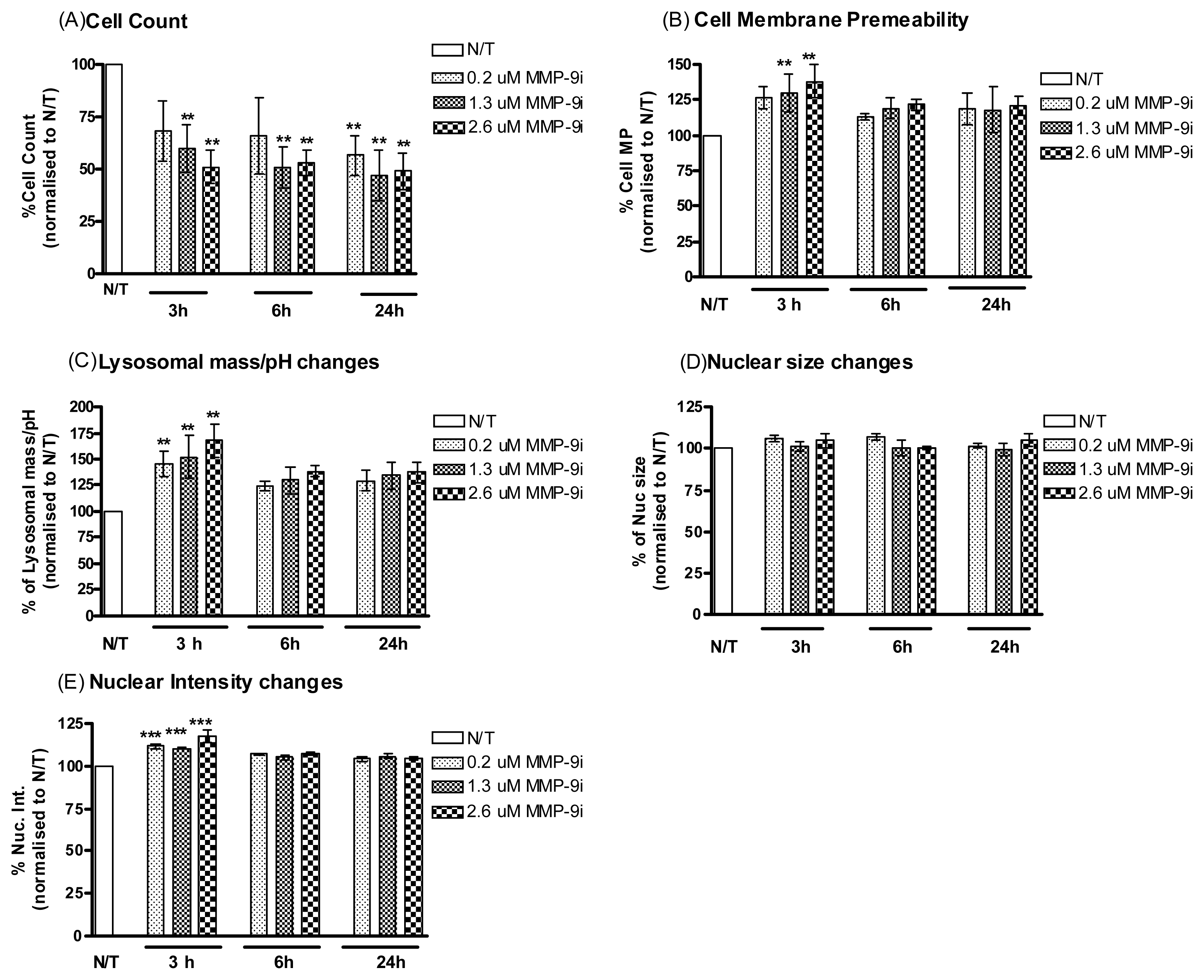
2.1.3. MMP-9/MMP-2i Induces Cell Death in Cisplatin Resistant Ovarian Cancer Cells
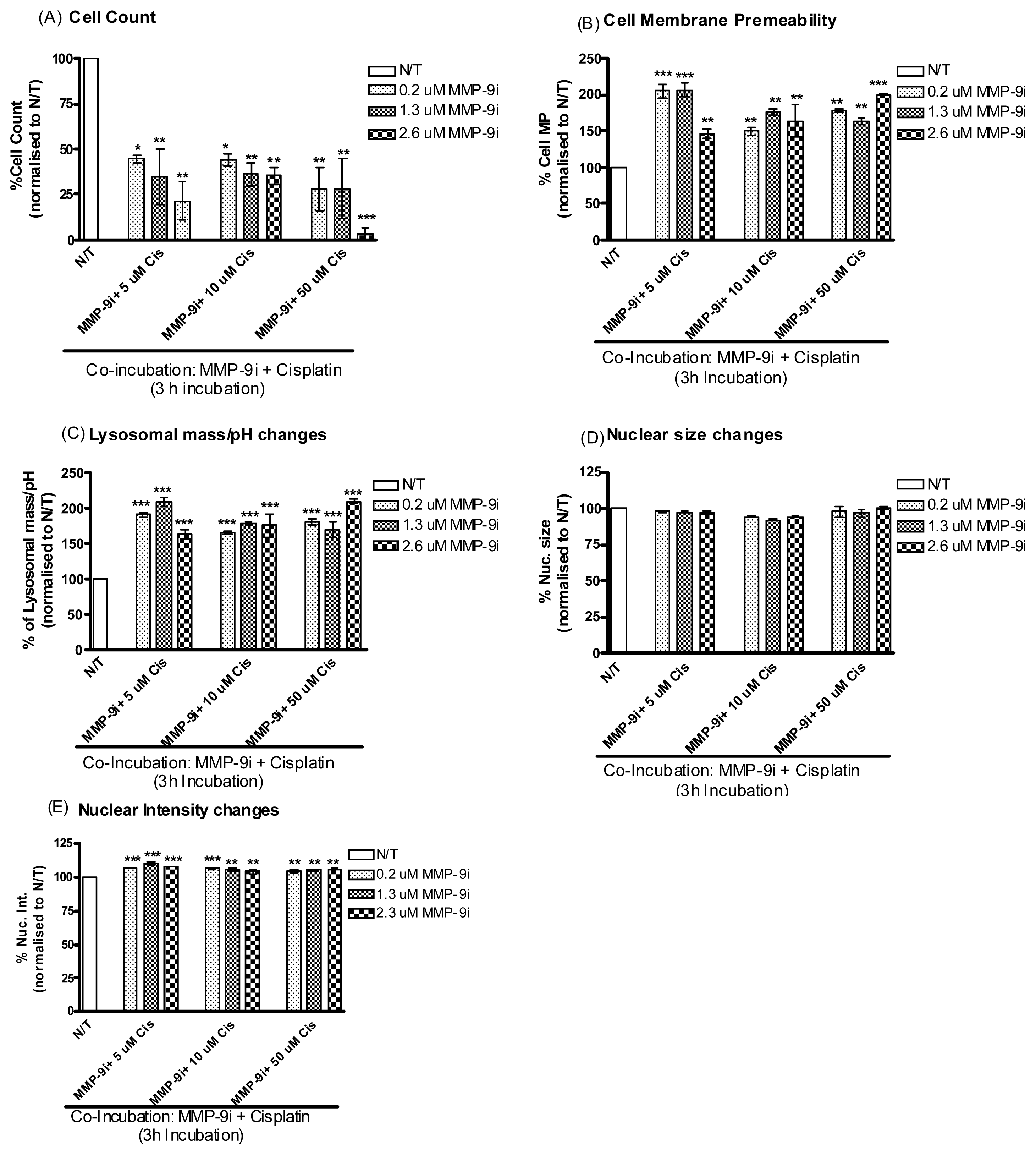
2.1.4. MMP-9/MMP-2i Enhances Cisplatin-Induced Cell Death in Chemoresistant Ovarian Cancer Cells at an Early Time Point
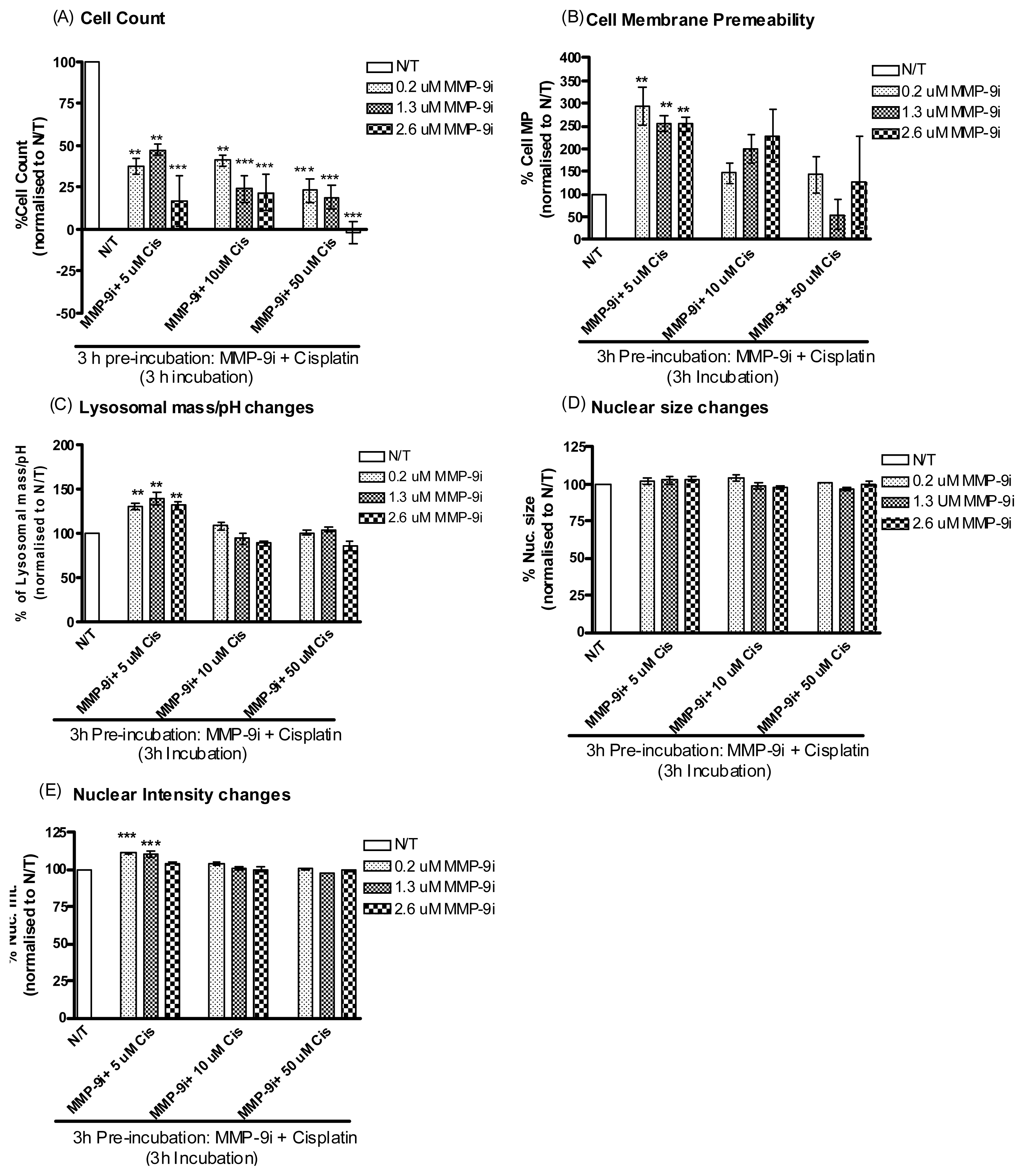
2.1.5. Pre-Incubation with MMP-9/MMP-2i further Enhanced Cisplatin Induced Cytotoxicity
2.1.6. MMP-9/MMP-2i and Cisplatin Induces Cell Death in Chemosensitive Ovarian Cancer Cells
2.1.7. Evaluation of MMP-9 Expression by Immunostaining in vivo
2.1.8. ELISA Analysis of Serum
2.2. Discussion
3. Experimental Section
3.1. Cell Culture
3.2. Preparation of Cell Lines for RNA Extraction
3.3. TaqMan PCR Analysis of Selected Candidates
3.4. Chemicals
3.5. Experimental Design
3.6. Multiparameter Cytotoxicity Assay Using HCS System
3.7. Immunohistochemical Staining and Analysis
3.8. Patient Serum Samples
3.9. ELISA for MMP-9 and Its Inhibitor TIMP-2
3.10. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-02085-s001.pdfAcknowledgements
- Authors’ ContributionsAL conceived and designed the study, carried out the cell line experiments/TaqMan analysis and drafted the manuscript. BMM and AD carried out the HCS analysis. LME, LK, LN, FAS and RL processed bloods and participated in the ELISA of the serum. BMM, ML, MR, RF, CD and SF performed the IHC staining and analysis. MG, CM and OS participated in the design and study coordination. NG and TD facilitated recruitment of patients and helped to draft the manuscript. JOL and SOT participated in the study design and drafted the manuscript. All authors read, edited and approved the final manuscript.
- Conflict of InterestThe authors declare no conflict of interest.
References
- Vasey, P.A. Resistance to chemotherapy in advanced ovarian cancer: Mechanisms and current strategies. Br. J. Cancer 2003, 89, S23–S28. [Google Scholar]
- Laios, A.; O’Toole, S.; Flavin, R.; Martin, C.; Ring, M.; Gleeson, N.; D’Arcy, T.; McGuinness, E.; Sheils, O.; Sheppard, B.; et al. An integrative model for recurrence in ovarian cancer. Mol. Cancer 2008, 7, 8–11. [Google Scholar]
- Laios, A.; O’Toole, S.; Flavin, R.; Martin, C.; Kelly, L.; Ring, M.; Finn, S.; Barrett, C.; Loda, M.; Gleeson, N.; et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol. Cancer 2008, 7. [Google Scholar] [CrossRef]
- Overall, C.M.; Lopez-Otin, C. Strategies for mmp inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar]
- Curran, S.; Murray, G.I. Matrix metalloproteinases in tumour invasion and metastasis. J. Pathol 1999, 189, 300–308. [Google Scholar]
- Karakiulakis, G.; Papanikolaou, C.; Jankovic, S.M.; Aletras, A.; Papakonstantinou, E.; Vretou, E.; Mirtsou-Fidani, V. Increased type IV collagen-degrading activity in metastases originating from primary tumors of the human colon. Invasion Metastasis 1997, 17, 158–168. [Google Scholar]
- Zucker, S.; Lysik, R.M.; Zarrabi, M.H.; Moll, U. Mr 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res 1993, 53, 140–146. [Google Scholar]
- Fishman, D.A.; Banionis, S.; Kearns, A.S.; Chilukuri, K.; Stack, M.S. Production of extracellular matrix-degrading proteinases by primary cultures of human epithelial ovarian carcinoma cells. Cancer 1997, 80, 1457–1463. [Google Scholar]
- Davidson, B.; Goldberg, I.; Gotlieb, W.; Kopolovic, J.; Ben-Baruch, G.; Nesland, J.; Berner, A.; Bryne, M.; Reich, R. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin. Exp. Metastasis 1999, 17, 799–808. [Google Scholar]
- Alshenawy, H.A. Immunohistochemical expression of epidermal growth factor receptor, E-cadherin, and matrix metalloproteinase-9 in ovarian epithelial cancer and relation to patient deaths. Ann. Diagn. Pathol 2010, 14, 387–395. [Google Scholar]
- Kamat, A.A.; Fletcher, M.; Gruman, L.M.; Mueller, P.; Lopez, A.; Landen, C.N.; Han, L.; Gershenson, D.M.; Sood, A.K. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin. Cancer Res 2006, 12, 1707–1714. [Google Scholar]
- Schmalfeldt, B.; Prechtel, D.; Härting, K.; Späthe, K.; Rutke, S.; Konik, E.; Fridman, R.; Berger, U.; Schmitt, M.; Kuhn, W.; et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin. Cancer Res 2001, 7, 2396–2404. [Google Scholar]
- Davidson, B. Ovarian carcinoma and serous effusions. Changing views regarding tumor progression and review of current literature. Anal. Cell Pathol 2002, 23, 107–128. [Google Scholar]
- Nemunaitis, J.; Poole, C.; Primrose, J.; Rosemurgy, A.; Malfetano, J.; Brown, P.; Berrington, A.; Cornish, A.; Lynch, K.; Rasmussen, H.; et al. Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: Selection of a biologically active and tolerable dose for longer-term studies. Clin. Cancer Res 1998, 4, 1101–1109. [Google Scholar]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer. J. Pathol 2002, 189, 300–308. [Google Scholar]
- Vogt, A.; Kalb, E.N.; Lazo, J.S. A scalable high-content cytotoxicity assay insensitive to changes in mitochondrial metabolic activity. Oncol. Res 2004, 14, 305–314. [Google Scholar]
- Mohamed, B.; Verma, N.; Prina-Mello, A.; Williams, Y.; Davies, A.M.; Bakos, G.; Tormey, L.; Edwards, C.; Hanrahan, J.; Salvati, A.; et al. Activation of stress-related signalling pathway in human cells upon SiO2 nanoparticles exposure as an early indicator of cytotoxicity. J. Nanobiotechnol. 2011, 9. [Google Scholar] [CrossRef]
- Byrne, F.; Prina-Mello, A.; Whelan, A.; Mohamed, B.M.; Davies, A.; Gun’ko, Y.K.; Coey, J.M.D.; Volkov, Y. High content analysis of the biocompatibility of nickel nanowires. J. Magn. Magn. Mater. 2009, 321, 1341–1345. [Google Scholar]
- Mohamed, B.M.; Verma, N.; Davies, A.M.; McGowan, M.; Staunton, K.C.; Prina-Mello, A.; Kelleher, D.; Botting, C.H.; Causey, C.P.; Thompson, P.R.; et al. Citrullination of proteins: A common post-translational modification pathway induced by different nanoparticles in vitro and in vivo. Nanomedicine 2012, 7, 1181–1195. [Google Scholar]
- Mohamed, B.M.; Feighery, C.; Williams, Y.; Davies, A.; Kelleher, D.; Volkov, Y.; Kelly, J.; Abuzakouk, M. The use of Cellomics to study enterocyte cytoskeletal proteins in coeliac disease patients. Cent. Eur. J. Biol 2008, 3, 258–267. [Google Scholar]
- Zhou, X.; Wong, S. Informatics challenges of high-throughput microscopy. IEEE Signal Process. Mag 2006, 23, 63–672. [Google Scholar]
- Long, A.; Volkov, Y. High content analysis approach for targeted gene silencing and probing nanoscale cell responses. Eur. Pharm. Rev 2009, 1, 22–30. [Google Scholar]
- Hu, X.; Li, D.; Zhang, W.; Zhou, J.; Tang, B.; Li, L. Matrix metallopoteinase-9 expression correlates with poor prognosis and involved in ovarian cancer cell invasion. Arch. Gynecol. Obstet 2012, 286, 1537–1543. [Google Scholar]
- Kwon, Y.; Cukierman, E.; Godwin, A.K. Differential expressions of adhesive molecules and proteases define mechanisms of ovarian tumour cell matrix penetration/invasion. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Sharma, S.; Santiskulvog, C.; Bentolola, L.A.; Rao, J.; Dorigo, O.; Gimzewski, J.K. Correlative nanomechanical profiling with super-resolution F-actin imaging reveals novel insights into mechanisms of cisplatin resistance in ovarian cancer cells. Nanomedicine 2012, 8, 757–766. [Google Scholar]
- Zhang, W.; Yang, H.-C.; Wang, Q.; Yang, Z.-J.; Chen, H.; Wang, S.-M.; Pan, Z.-M.; Tang, B.-J.; Li, Q.Q.; Li, L. Clinical value of combined detection of serum matrix metalloproteinase-9, heparanase, and cathepsin for determining ovarian cancer invasion and metastasis. Anticancer Res 2011, 31, 3423–3428. [Google Scholar]
- Gershtein, E.S.; Levkina, N.V.; Digayeva, M.A.; Laktionov, K.P.; Tereshkina, I.V.; Kushlinsky, N.E. Matrix metalloproteinases 2, 7, and 9 and tissue inhibitor of metalloproteinases-1 in tumors and serum of patients with ovarian neoplasms. Bull. Exp. Biol. Med 2010, 149, 628–631. [Google Scholar]
- Iizasa, T.; Fujisawa, T.; Suzuki, M.; Motohashi, S.-i.; Yasufuku, K.; Yasukawa, T.; Baba, M.; Shiba, M. Elevated levels of circulating plasma matrix metalloproteinase 9 in non-small cell lung cancer patients. Clin. Cancer Res 1999, 5, 149–153. [Google Scholar]
- Devy, L.; Dransfield, D.T. New strategies for the next generation of matrix-metalloproteinase inhibitors: Selectively targeting membrane-anchored mmps with therapeutic antibodies. Biochem. Res. Int. 2011, 2011. [Google Scholar] [CrossRef]
- Lubbe, W.J.; Zhou, Z.Y.; Fu, W.; Zuzga, D.; Schulz, S.; Fridman, R.; Muschel, R.J.; Waldman, S.A.; Pitari, G.M. Tumor epithelial cell matrix metalloproteinase 9 is a target for antimetastatic therapy in colorectal cancer. Clin. Cancer Res 2006, 12, 1876–1882. [Google Scholar]
- Bond, M.; Murphy, G.; Bennett, M.R.; Amour, A.; Knäuper, V.; Newby, A.C.; Baker, A.H. Localization of the death domain of tissue inhibitor of metalloproteinase-3 to the n terminus. J. Biol. Chem 2000, 275, 41358–41363. [Google Scholar]
- Bendeck, M.P.; Irvin, C.; Reidy, M.A. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ. Res 1996, 78, 38–43. [Google Scholar]
- Southgate, K.M.; Davies, M.B.R.; Newby, A.C. Involvement of extracellular-matrix-degrading metalloproteinases in rabbit aortic smooth-muscle cell proliferation. Biochem. J 1992, 288, 93–99. [Google Scholar]
- Karam, A.K.; Santiskulvong, C.; Fekete, M.; Zabih, S.; Eng, C.; Dorigo, O. Cisplatin and PI3kinase inhibition decrease invasion and migration of human ovarian carcinoma cells and regulate matrix-metalloproteinase expression. Cytoskeleton 2010, 67, 535–544. [Google Scholar]
- Tamura, Y.; Watanabe, F.; Nakatani, T.; Yasui, K.; Fuji, M.; Komurasaki, T.; Tsuzuki, H.; Maekawa, R.; Yoshioka, T.; Kawada, K.; et al. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J. Med. Chem 1998, 41, 640–649. [Google Scholar]
- Ikeda, M.; Maekawa, R.; Tanaka, H.; Matsumoto, M.; Takeda, Y.; Tamura, Y.; Nemori, R.; Yoshioka, T. Inhibition of gelatinolytic activity in tumor tissues by synthetic matrix metalloproteinase inhibitor: Application of film in situ zymography. Clin. Cancer Res 2000, 6, 3290–3296. [Google Scholar]
- Nyormoi, O.; Mills, L.; Bar-Eli, M. An MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell Death Differ 2003, 10, 558–569. [Google Scholar]
- Rowinsky, E.K.; Humphrey, R.; Hammond, L.A.; Aylesworth, C.; Smetzer, L.; Hidalgo, M.; Morrow, M.; Smith, L.; Garner, A.; Sorensen, J.M.; et al. Phase I and pharmacologic study of the specific matrix metalloproteinase inhibitor bay 12-9566 on a protracted oral daily dosing schedule in patients with solid malignancies. J. Clin. Oncol 2000, 18, 178–186. [Google Scholar]
- Kilian, M.; Gregor, J.I.; Heukamp, I.; Hanel, M.; Ahlgrimm, M.; Schimke, I.; Kristiansen, G.; Ommer, A.; Walz, M.K.; Jacobi, C.A.; et al. Matrix metalloproteinase inhibitor RO 28-2653 decreases liver metastasis by reduction of MMP-2 and MMP-9 concentration in bop-induced ductal pancreatic cancer in syrian hamsters: Inhibition of matrix metalloproteinases in pancreatic cancer. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 429–434. [Google Scholar]
- Mohamed, B.; Feighery, C.; Kelly, J.; Coates, C.; O’Shea, U.; Barnes, L.; Abuzakouk, M. Increased protein expression of matrix metalloproteinases -1, -3, and -9 and TIMP-1 in patients with gluten-sensitive enteropathy. Dig. Dis. Sci 2006, 51, 1862–1868. [Google Scholar]
- Boehm, J.S.; Hahn, W.C. Understanding transformation: Progress and gaps. Curr. Opin. Genet. Dev 2005, 15, 13–17. [Google Scholar]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul 1984, 22, 27–55. [Google Scholar]
- Bliss, C. The toxicity of poisons applied jointly. Ann. Appl. Biol 1939, 26, 585–615. [Google Scholar]


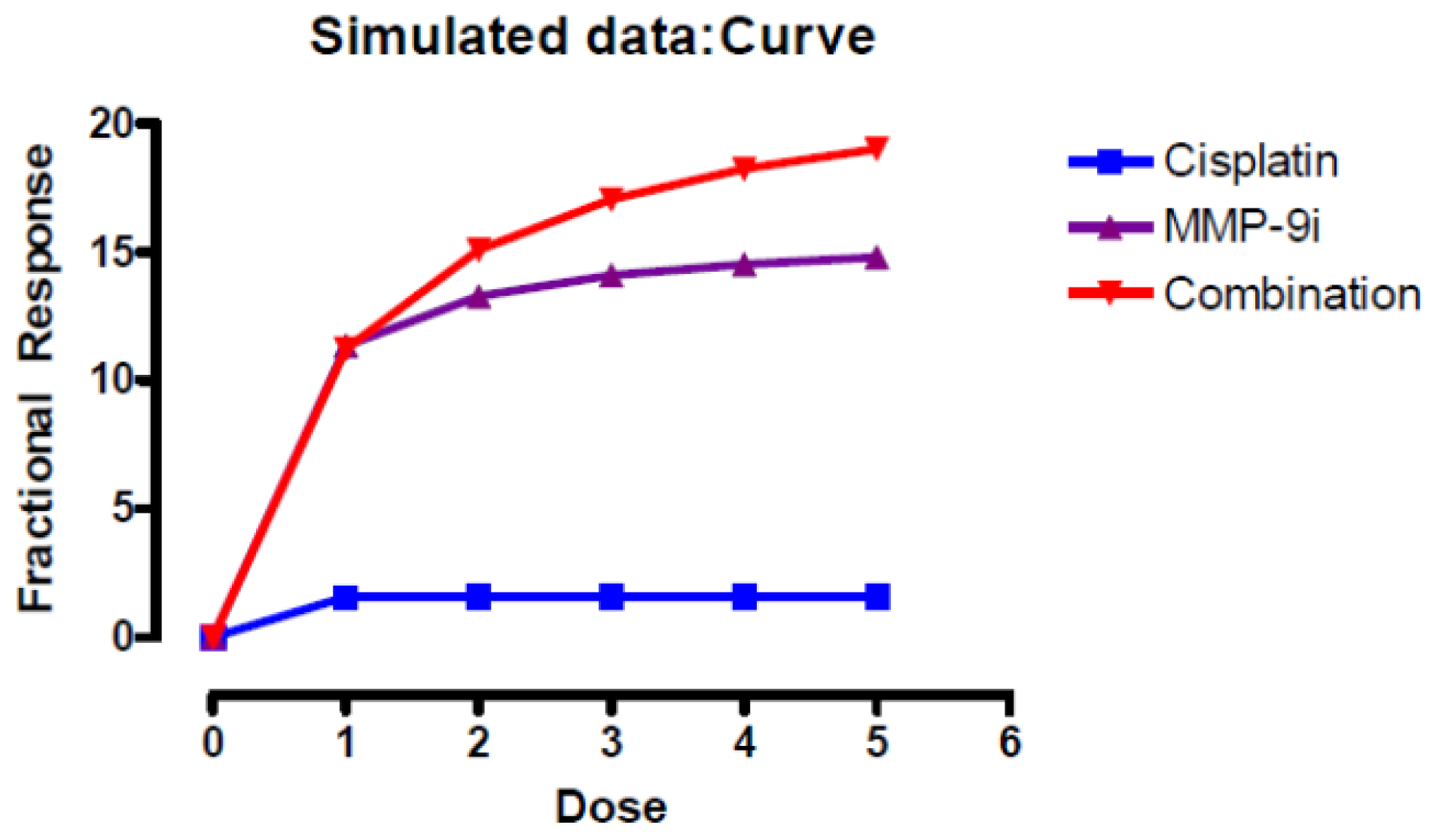
| Time | Cisplatin concentration μM | MMP-9/MMP-2i concentration μM | ||||
|---|---|---|---|---|---|---|
| 5 | 10 | 50 | 0.2 | 1.3 | 2.6 | |
| 3 | 8 | 6 | 8 | 32 | 40 ** | 49 ** |
| 6 | +3 | 9 | 30 | 34 | 49 ** | 47 ** |
| 24 | 47 ** | 60 ** | 66 ** | 44 ** | 53 ** | 51 ** |
| Conc cis μM | Co-incubation 3 h Conc MMP-9/MMP-2i μM | Pre-incubation 3 h Conc MMP-9/MMP-2i μM | ||||
| 2 | 1.3 | 2.6 | 0.2 | 1.3 | 2.6 | |
| 5 | 55 * | 65 ** | 79 ** | 62 ** | 53 ** | 83 *** |
| 10 | 56 * | 44 ** | 65 ** | 59 ** | 76 *** | 78 *** |
| 50 | 72 ** | 72 ** | 97 *** | 77 *** | 81 *** | 100 *** |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Laios, A.; Mohamed, B.M.; Kelly, L.; Flavin, R.; Finn, S.; McEvoy, L.; Gallagher, M.; Martin, C.; Sheils, O.; Ring, M.; et al. Pre-Treatment of Platinum Resistant Ovarian Cancer Cells with an MMP-9/MMP-2 Inhibitor Prior to Cisplatin Enhances Cytotoxicity as Determined by High Content Screening. Int. J. Mol. Sci. 2013, 14, 2085-2103. https://doi.org/10.3390/ijms14012085
Laios A, Mohamed BM, Kelly L, Flavin R, Finn S, McEvoy L, Gallagher M, Martin C, Sheils O, Ring M, et al. Pre-Treatment of Platinum Resistant Ovarian Cancer Cells with an MMP-9/MMP-2 Inhibitor Prior to Cisplatin Enhances Cytotoxicity as Determined by High Content Screening. International Journal of Molecular Sciences. 2013; 14(1):2085-2103. https://doi.org/10.3390/ijms14012085
Chicago/Turabian StyleLaios, Alexandros, Bashir M. Mohamed, Lynne Kelly, Richard Flavin, Stephen Finn, Lynda McEvoy, Michael Gallagher, Cara Martin, Orla Sheils, Martina Ring, and et al. 2013. "Pre-Treatment of Platinum Resistant Ovarian Cancer Cells with an MMP-9/MMP-2 Inhibitor Prior to Cisplatin Enhances Cytotoxicity as Determined by High Content Screening" International Journal of Molecular Sciences 14, no. 1: 2085-2103. https://doi.org/10.3390/ijms14012085