Induction of Heat Shock Protein 70 Ameliorates Ultraviolet-Induced Photokeratitis in Mice
Abstract
:1. Introduction
2. Results and Discussion
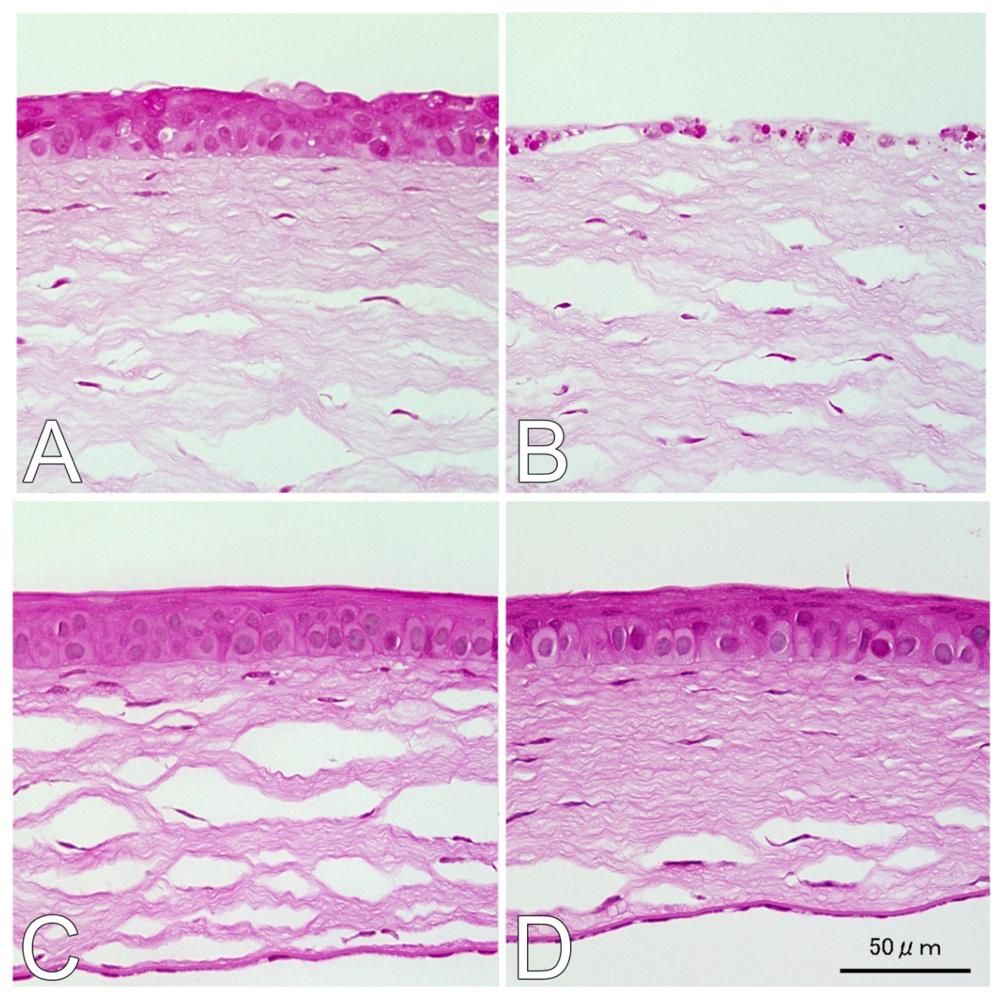
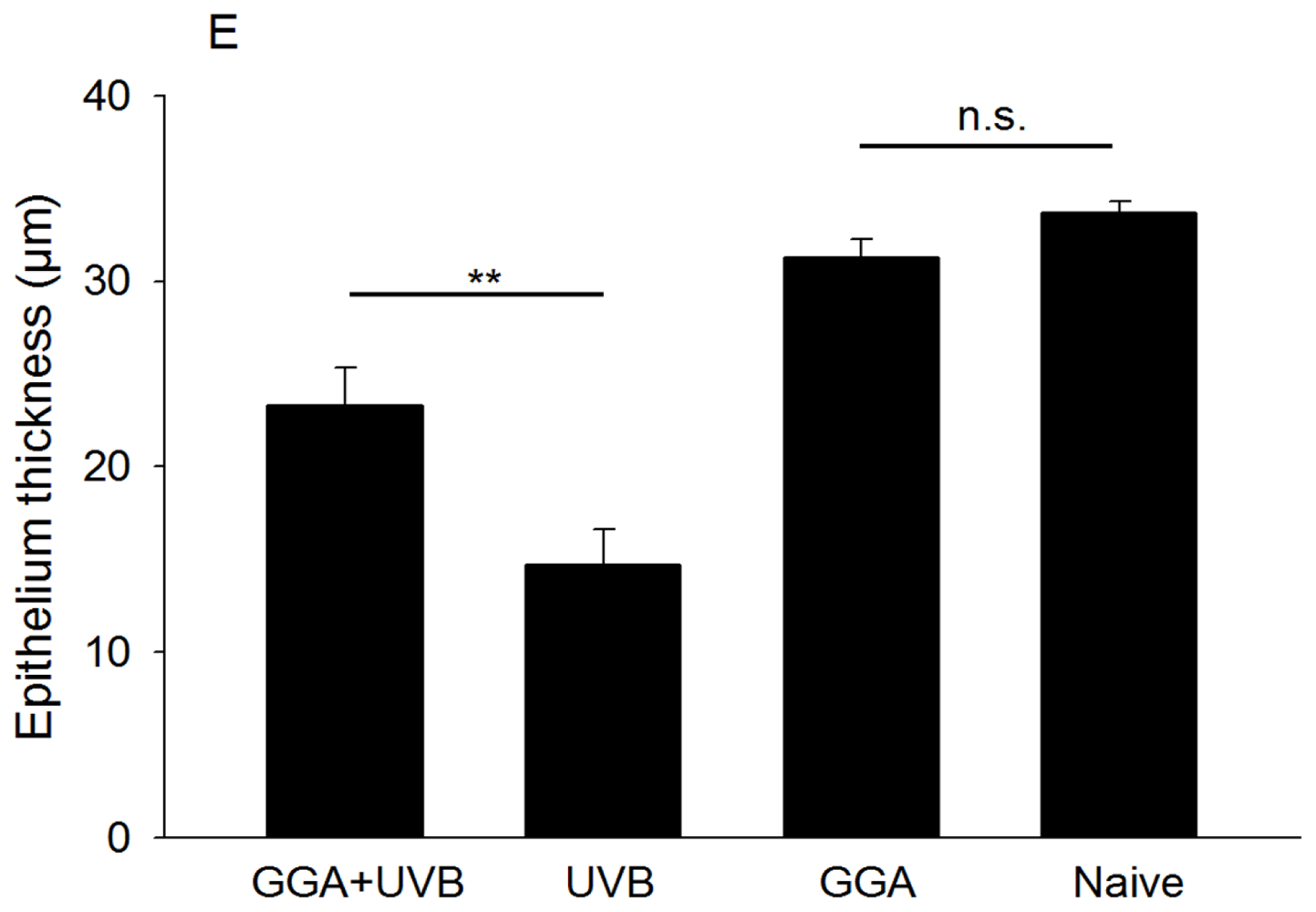
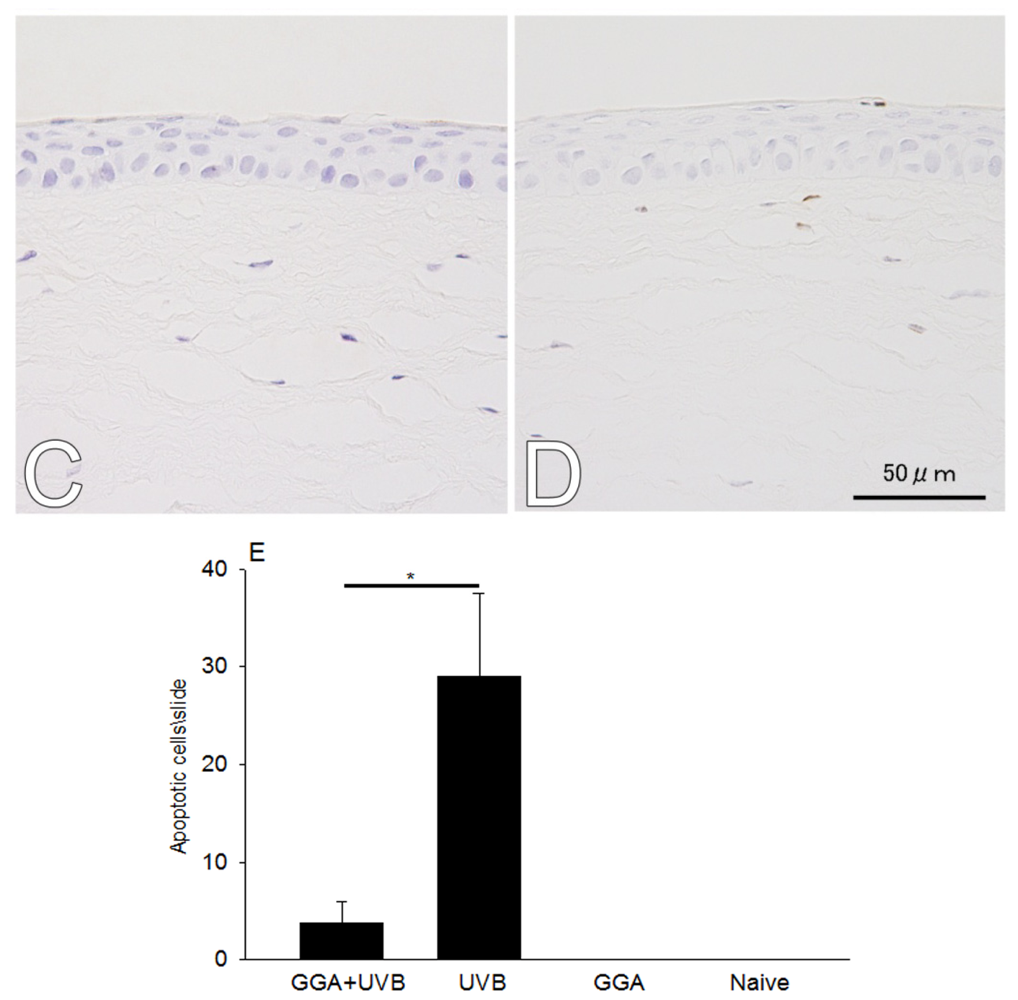
2.1. Hematoxylin and Eosin (H&E) and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining
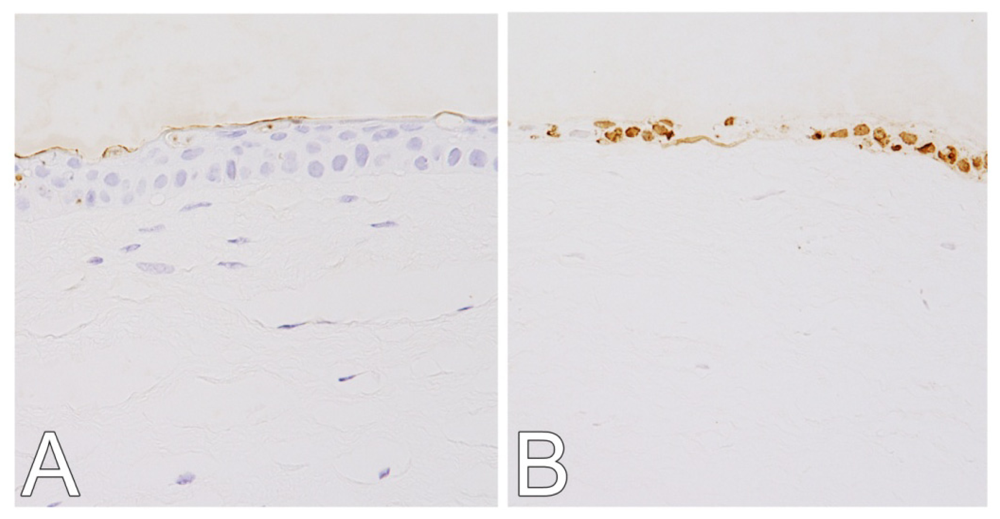
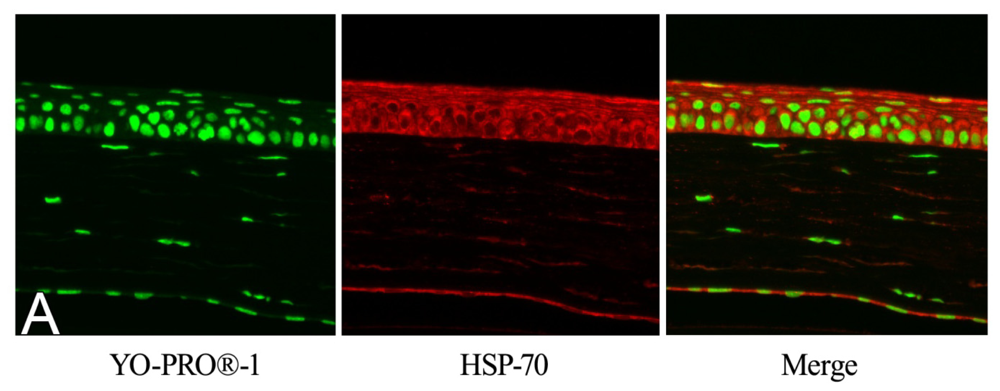
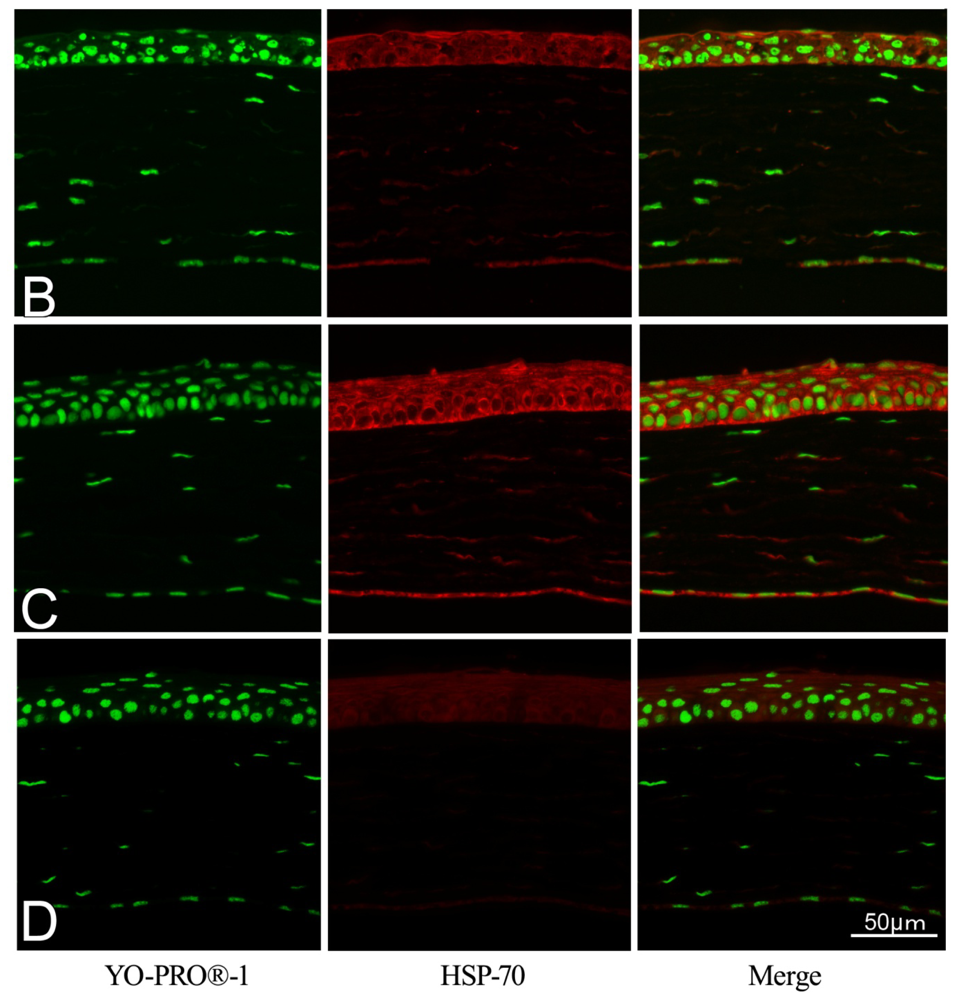
2.2. Effect of GGA on HSP70 Expression
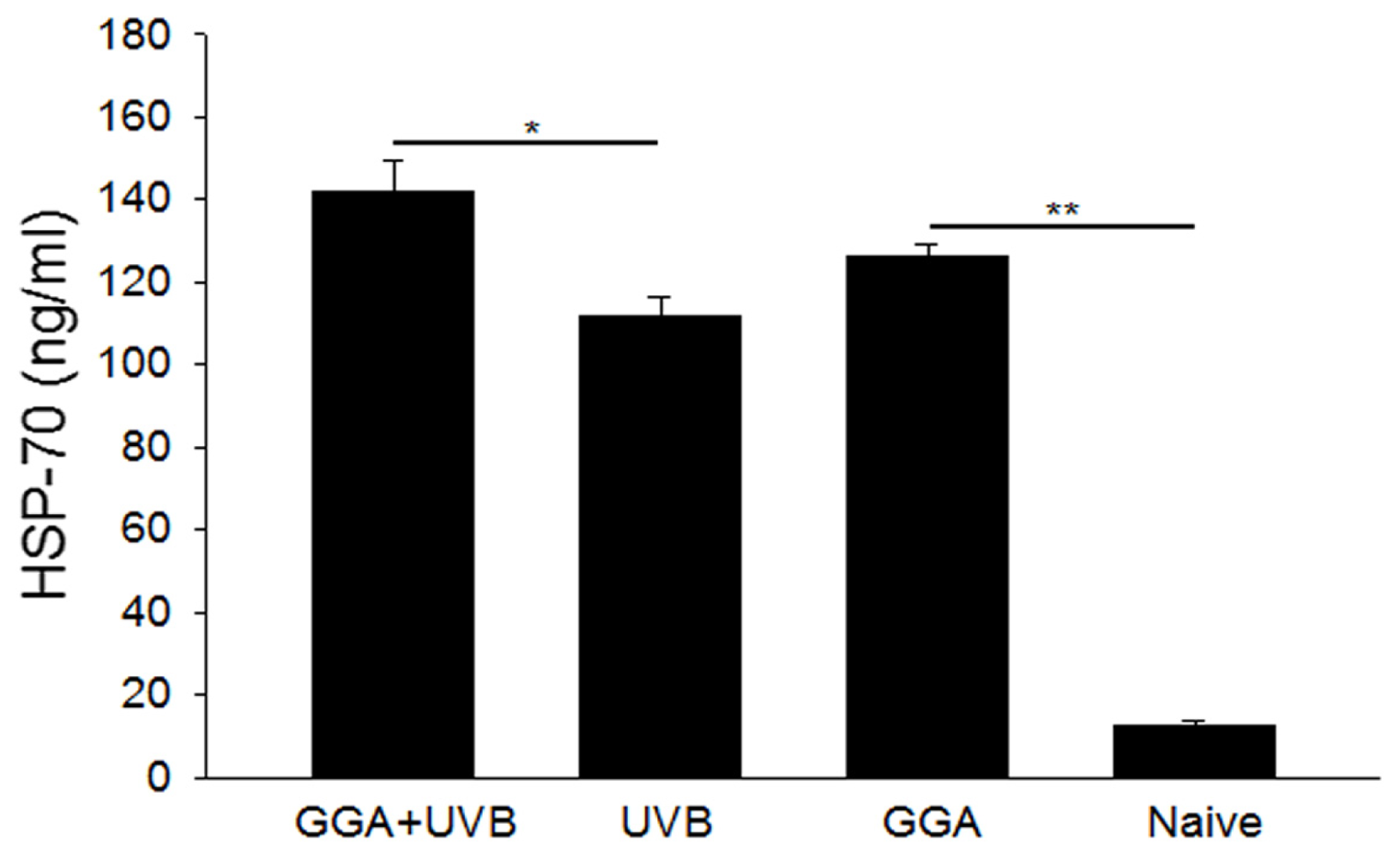
2.3. Quantification of HSP70 Levels
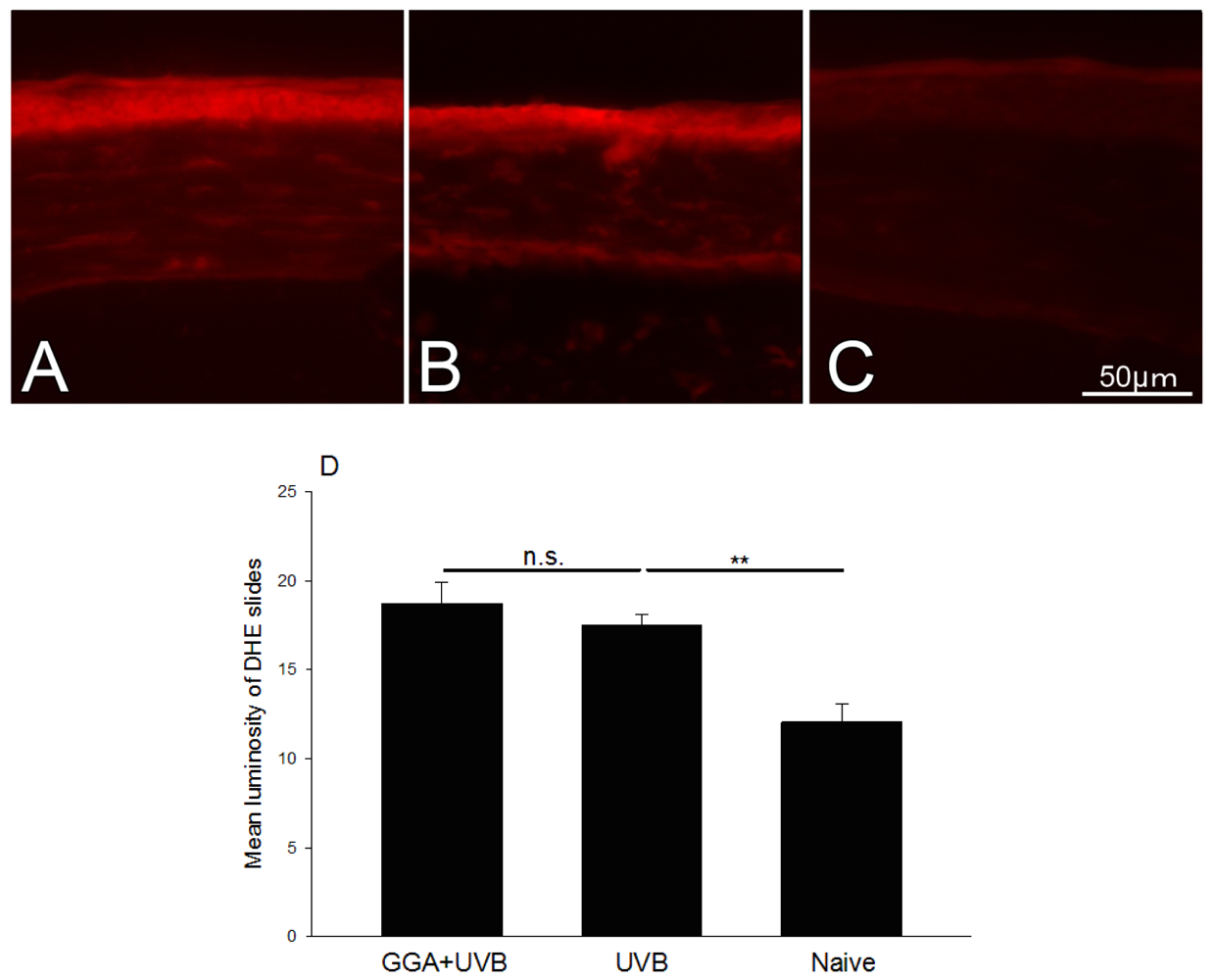
2.4. Detection of ROS
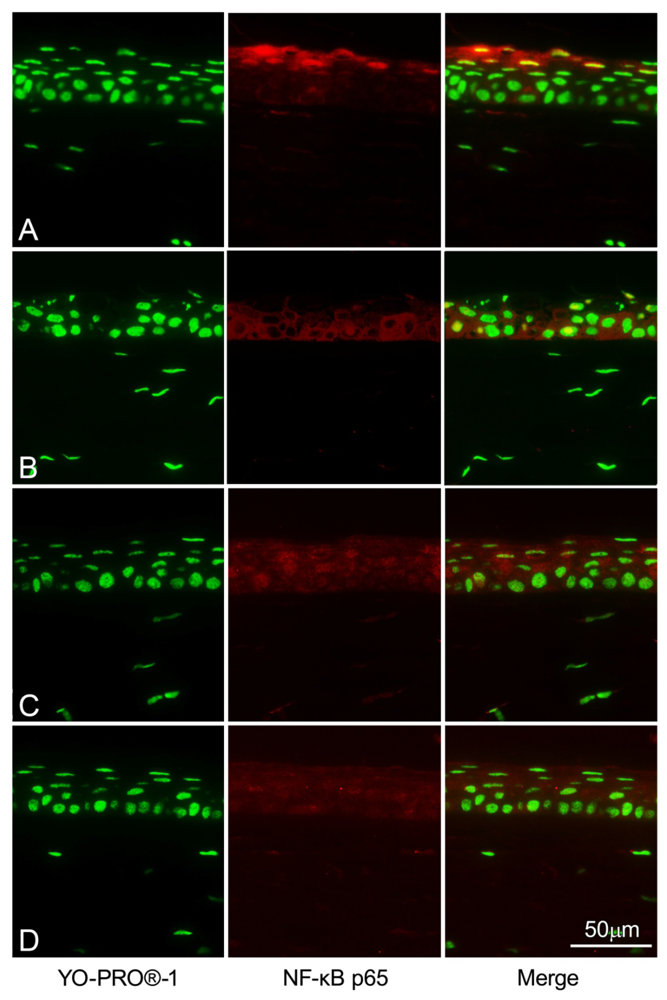
2.5. NF-κB Immunohistochemistry Staining
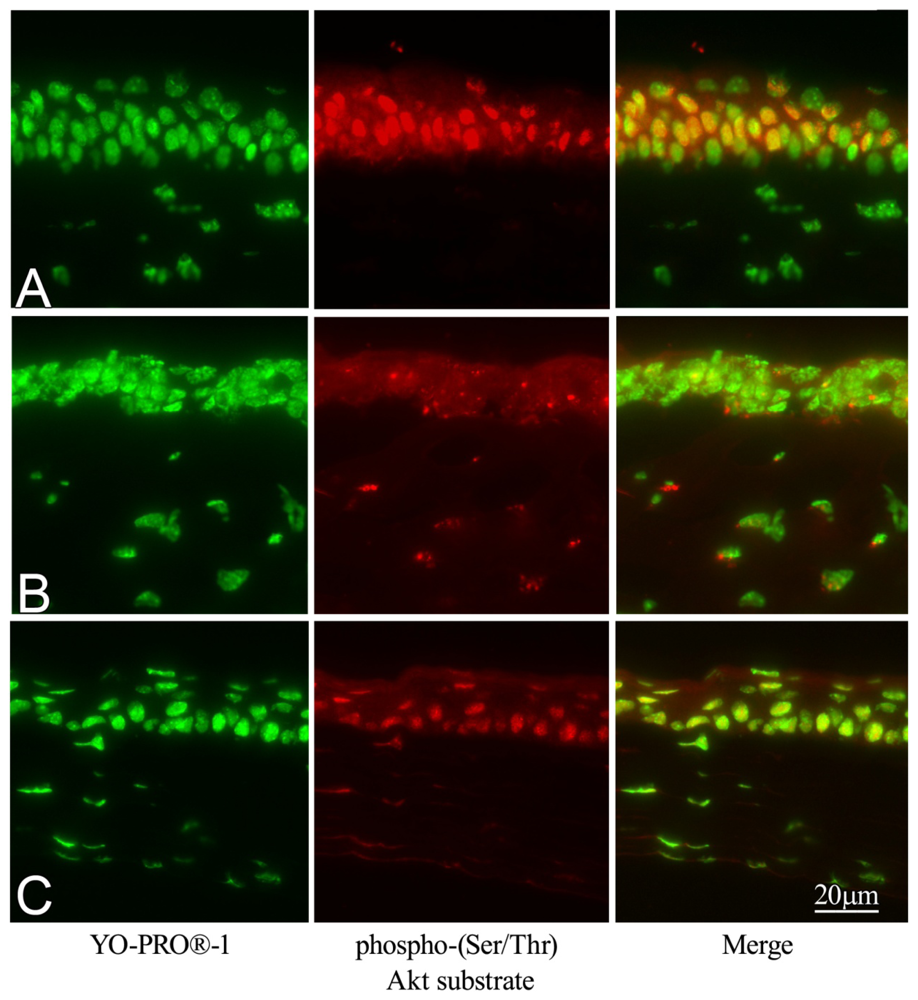
2.6. Effect of GGA on Akt Phosphorylation
3. Discussion
4. Experimental Section
4.1. Animals and Reagents
4.2. UV Irradiation and Sample Collection
4.3. H&E and TUNEL Staining
4.4. Immunohistochemical Staining for HSP70 and Akt Substrate in Corneal Tissue
4.5. Quantifying HSP70 with Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Detection of Reactive Oxygen Species (ROS)
4.7. NF-κB Immunohistochemistry Staining
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Schein, O.D. Phototoxicity and the cornea. J. Natl. Med. Assoc 1992, 84, 579–583. [Google Scholar]
- Cullen, A.P.; Chou, B.R.; Hall, M.G.; Jany, S.E. Ultraviolet-B damages corneal endothelium. Am. J. Optom. Physiol. Opt 1984, 61, 473–478. [Google Scholar]
- Bilski, J.; Sarosiek, J.; Merty, V.L.; Aono, M.; Moriga, M.; Slomiany, A.; Slomiany, B.L. Enhancement of the lipid content and physical properties of gastric mucus by geranylgeranylacetone. Biochem. Pharmacol. 1987, 36, 4059–4065. [Google Scholar]
- Ooie, T.; Takahashi, N.; Saikawa, T.; Nawata, T.; Arikawa, M.; Yamanaka, K.; Hara, M.; Shimada, T.; Sakata, T. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation 2001, 104, 1837–1843. [Google Scholar]
- Missotten, G.S.; Journee-de Korver, J.G.; de Wolff-Rouendaal, D.; Keunen, J.E.; Schlingemann, R.O.; Jager, M.J. Heat shock protein expression in the eye and in uveal melanoma. Invest. Ophthalmol. Vis. Sci 2003, 44, 3059–3065. [Google Scholar]
- Harada, C.; Nakamura, K.; Guo, X.; Kitaichi, N.; Mitamura, Y.; Yoshida, K.; Ohno, S.; Yoshida, H.; Harada, T. Neuroprotective effect of geranylgeranylacetone against ischemia-induced retinal injury. Mol. Vis 2007, 13, 1601–1607. [Google Scholar]
- Kitamei, H.; Kitaichi, N.; Yoshida, K.; Nakai, A.; Fujimoto, M.; Kitamura, M.; Iwabuchi, K.; Miyazaki, A.; Namba, K.; Ohno, S.; Onoe, K. Association of heat shock protein 70 induction and the amelioration of experimental autoimmune uveoretinitis in mice. Immunobiology 2007, 212, 11–18. [Google Scholar]
- Hirsch, C.; Gauss, R.; Sommer, T. Coping with stress: Cellular relaxation techniques. Trends Cell Biol 2006, 16, 657–663. [Google Scholar]
- Beckmann, R.P.; Mizzen, L.E.; Welch, W.J. Interaction of Hsp 70 with newly synthesized proteins: Implications for protein folding and assembly. Science 1990, 248, 850–854. [Google Scholar]
- van Molle, W.; Wielockx, B.; Mahieu, T.; Takada, M.; Taniguchi, T.; Sekikawa, K.; Libert, C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity 2002, 16, 685–695. [Google Scholar]
- Barbe, M.F.; Tytel, M.; Gower, D.J.; Welch, W.J. Hyperthermia protects against light damage in rat retina. Science 1988, 241, 1817–1820. [Google Scholar]
- Brachmann, C.B.; Jassim, O.W.; Wachsmuth, B.D.; Cagan, R.L. The Drosophila bcl-2 family member dBorg-1 functions in the apoptotic response to UV-irradiation. Curr. Biol 2000, 10, 547–550. [Google Scholar]
- Kayama, M.; Nakazawa, T.; Thanos, A.; Morizane, Y.; Murakami, Y.; Theodoropoulou, S.; Abe, T.; Vavvas, D.; Miller, J.W. Heat shock protein 70 (HSP70) is critical for the photoreceptor stress response after retinal detachment via modulating anti-apoptotic Akt kinase. Am. J. Pathol 2011, 178, 1080–1091. [Google Scholar]
- Pauloin, T.; Dutot, M.; Joly, F.; Warnet, J.M.; Rat, P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol. Vis 2009, 15, 577–583. [Google Scholar]
- Lennikov, A.; Kitaichi, N.; Fukase, R.; Murata, M.; Noda, K.; Ando, R.; Ohguchi, T.; Kawakita, T.; Ohno, S.; Ishida, S. Amelioration of ultraviolet-induced photokeratitis in mice treated with astaxanthin eye drops. Mol. Vis 2012, 18, 455–464. [Google Scholar]
- Senf, S.M.; Dodd, S.L.; McClung, J.M.; Judge, A.R. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 2008, 22, 3836–3845. [Google Scholar]
- Chen, H.; Wu, Y.; Zhang, Y.; Jin, L.; Luo, L.; Xue, B.; Lu, C.; Zhang, X.; Yin, Z. Hsp70 inhibits lipopolysaccharide-induced NF-kappaB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett 2006, 580, 3145–3152. [Google Scholar]
- Guzhova, I.V.; Darieva, Z.A.; Melo, A.R.; Margulis, B.A. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones 1997, 2, 132–139. [Google Scholar]
- Alexander, G.; Carlsen, H.; Blomhoff, R. Corneal NF-kappaB activity is necessary for the retention of transparency in the cornea of UV-B-exposed transgenic reporter mice. Exp. Eye Res 2006, 82, 700–709. [Google Scholar]
- Amin, M.; Haas, C.; Zhu, K.; Mansfield, P.; Kim, M.; Lackowski, N.; Koch, A. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood 2006, 107, 2252–2261. [Google Scholar]
- Kitaichi, N.; Shimizu, T.; Yoshida, K.; Honda, A.; Yoshihisa, Y.; Kase, S.; Ohgami, K.; Norisugi, O.; Makino, T.; Nishihira, J.; Yamagishi, S.; Ohno, S. Macrophage migration inhibitory factor ameliorates UV-induced photokeratitis in mice. Exp. Eye Res 2008, 86, 929–935. [Google Scholar]
- Kukner, A.; Colakoglu, N.; Serin, D.; Alagoz, G.; Celebi, S.; Kukner, A.S. Effects of intraperitoneal vitamin E, melatonin and aprotinin on leptin expression in the guinea pig eye during experimental uveitis. Acta Ophthalmol. Scand 2006, 84, 54–61. [Google Scholar]
- Burton, G.W.; Traber, M.G. Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annu. Rev. Nutr 1990, 10, 357–382. [Google Scholar]
- Takaki, E.; Fujimoto, M.; Sugahara, K.; Nakahari, T.; Yonemura, S.; Tanaka, Y.; Hayashida, N.; Inouye, S.; Takemoto, T.; Yamashita, H.; Nakai, A. Maintenance of olfactory neurogenesis requires HSF1, a major heat shock transcription factor in mice. J. Biol. Chem 2006, 281, 4931–4937. [Google Scholar]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar]
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lennikov, A.; Kitaichi, N.; Kase, S.; Noda, K.; Horie, Y.; Nakai, A.; Ohno, S.; Ishida, S. Induction of Heat Shock Protein 70 Ameliorates Ultraviolet-Induced Photokeratitis in Mice. Int. J. Mol. Sci. 2013, 14, 2175-2189. https://doi.org/10.3390/ijms14012175
Lennikov A, Kitaichi N, Kase S, Noda K, Horie Y, Nakai A, Ohno S, Ishida S. Induction of Heat Shock Protein 70 Ameliorates Ultraviolet-Induced Photokeratitis in Mice. International Journal of Molecular Sciences. 2013; 14(1):2175-2189. https://doi.org/10.3390/ijms14012175
Chicago/Turabian StyleLennikov, Anton, Nobuyoshi Kitaichi, Satoru Kase, Kousuke Noda, Yukihiro Horie, Akira Nakai, Shigeaki Ohno, and Susumu Ishida. 2013. "Induction of Heat Shock Protein 70 Ameliorates Ultraviolet-Induced Photokeratitis in Mice" International Journal of Molecular Sciences 14, no. 1: 2175-2189. https://doi.org/10.3390/ijms14012175



