Overlapping ATP2C1 and ASTE1 Genes in Human Genome: Implications for SPCA1 Expression?
Abstract
:1. Introduction
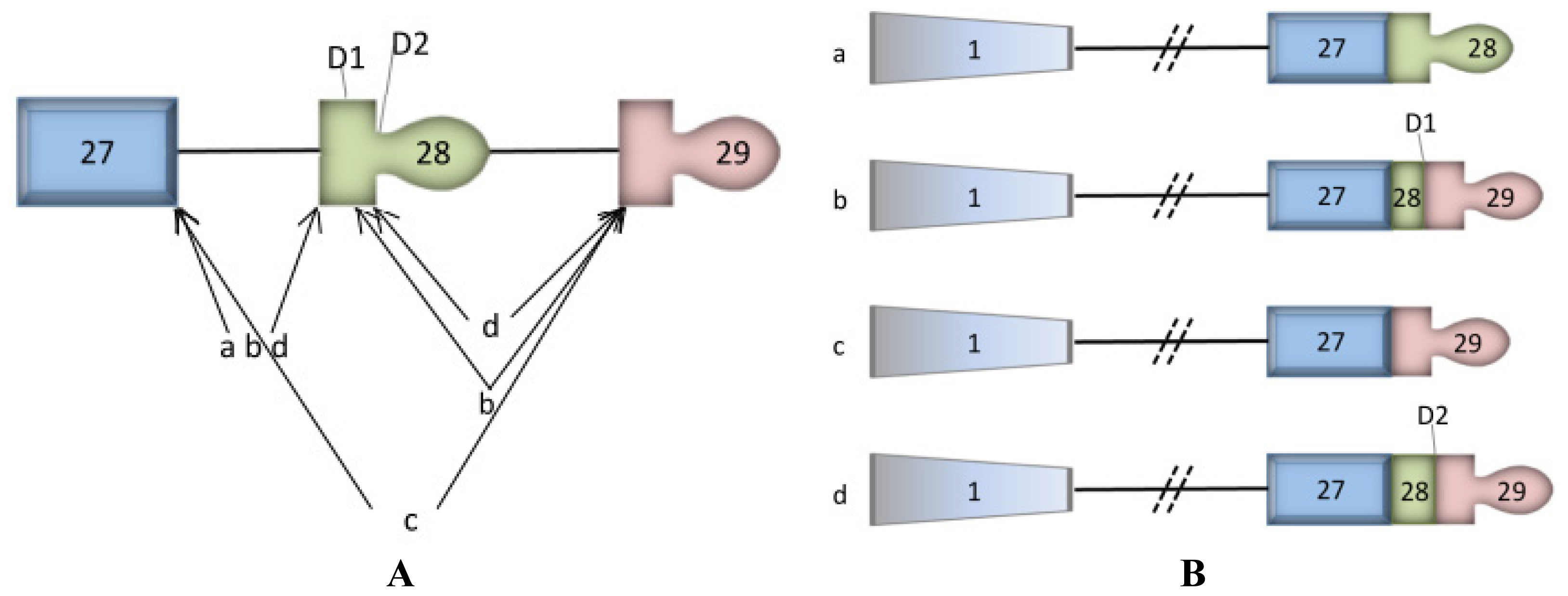
2. Mutations in ATP2C1 Cause Hailey-Hailey Disease in Humans but not Mice
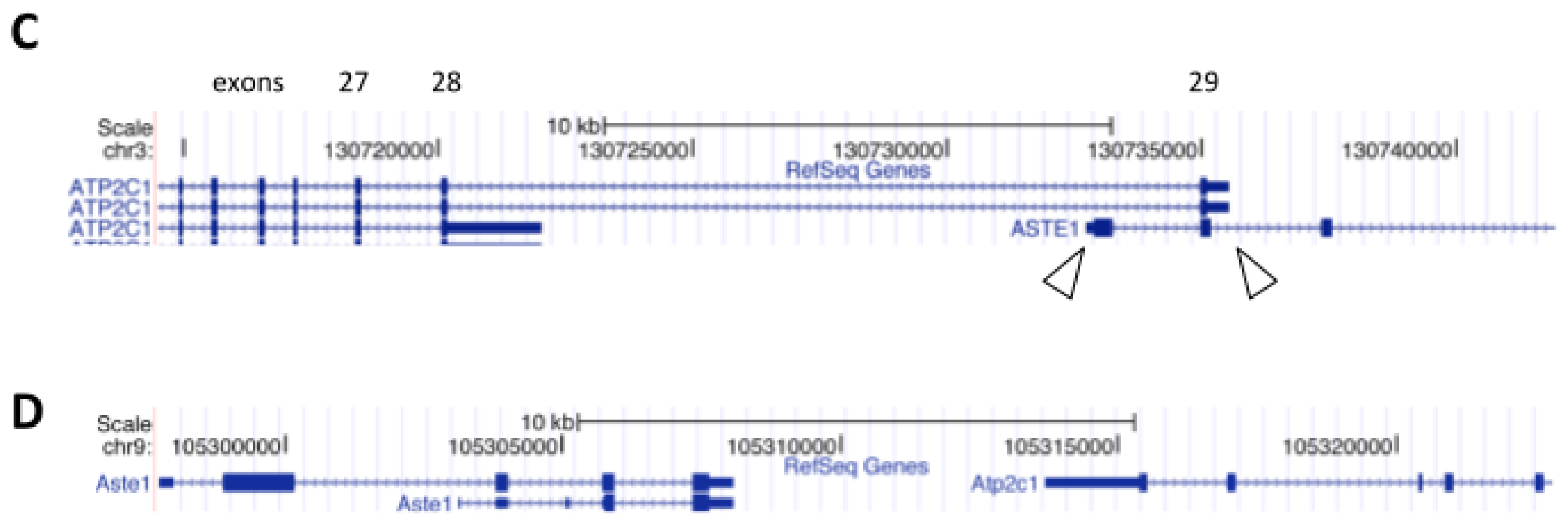
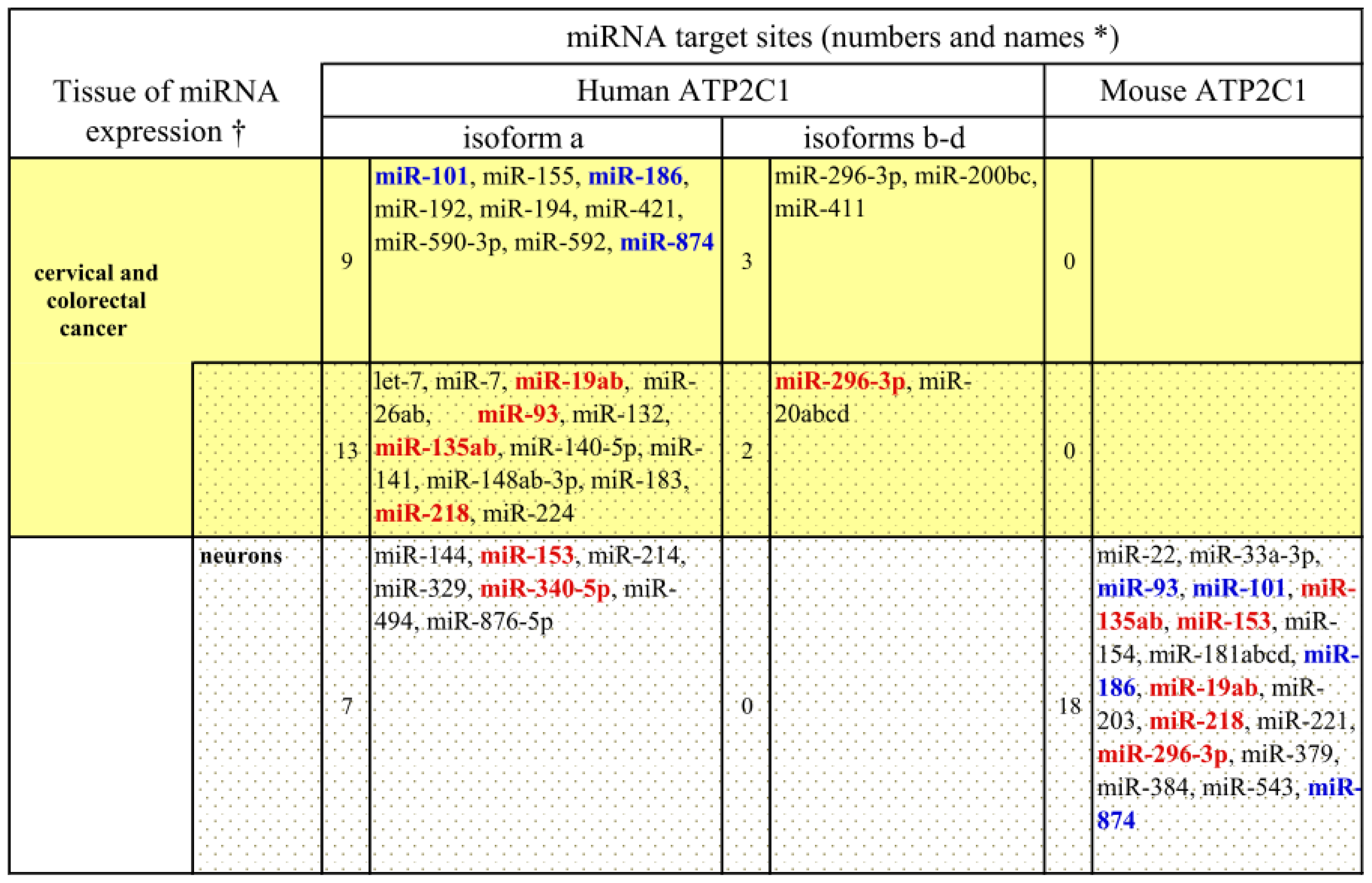
3. ASTE1 may Influence Expression of ATP2C1 Isoforms
4. Conclusions
Acknowledgements
Abbreviations
| Ca2+ | calcium ions |
| HHD | Hailey-Hailey disease |
| SPCA1 | secretory pathway (Ca2+)-ATPase pump type 1 |
| ROS | reactive oxygen species |
| TGN | trans-Golgi network |
- Conflict of InterestThe authors declare no conflict of interest.
References
- Fairclough, R.J.; Dode, L.; Vanoevelen, J.; Andersen, J.P.; Missiaen, L.; Raeymaekers, L.; Wuytack, F.; Hovnanian, A. Effect of Hailey-Hailey disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1). J. Biol. Chem 2003, 278, 24721–24730. [Google Scholar]
- Dode, L.; Andersen, J.P.; Vanoevelen, J.; Raeymaekers, L.; Missiaen, L.; Vilsen, B.; Wuytack, F. Functional comparison between secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and sarcoplasmic reticulum Ca2+-ATPase (SERCA) 1 isoforms by steady-state and transient kinetic analyses. J. Biol. Chem 2005, 280, 39124–39134. [Google Scholar]
- Sepúlveda, M.R.; Marcos, D.; Berrocal, M.; Raeymaekers, L.; Mata, A.M.; Wuytack, F. Activity and localization of the secretory pathway Ca2+-ATPase isoform 1 (SPCA1) in different areas of the mouse brain during postnatal development. Mol. Cell. Neurosci 2008, 38, 461–473. [Google Scholar]
- Strausberg, R.L.; Feingold, E.A.; Grouse, L.H.; Derge, J.G.; Klausner, R.D.; Collins, F.S.; Wagner, L.; Shenmen, C.M.; Schuler, G.D.; Altschul, S.F.; et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc. Natl. Acad. Sci. USA 2002, 99, 16899–16903. [Google Scholar]
- Hu, Z.; Bonifas, J.M.; Beech, J.; Bench, G.; Shigihara, T.; Ogawa, H.; Ikeda, S.; Mauro, T.; Epstein, E.H., Jr. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat. Genet. 2000, 24, 61–65. [Google Scholar]
- Sudbrak, R.; Brown, J.; Dobson-Stone, C.; Carter, S.; Ramser, J.; White, J.; Healy, E.; Dissanayake, M.; Larregue, M.; Perrussel, M.; et al. Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca2+ pump. Hum. Mol. Genet 2000, 9, 1131–1140. [Google Scholar]
- Behne, M.J.; Tu, C.L.; Aronchik, I.; Epstein, E.; Bench, G.; Bikle, D.D.; Pozzan, T.; Mauro, T.M. Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J. Invest. Dermatol 2003, 121, 688–694. [Google Scholar]
- Missiaen, L.; Raeymaekers, L.; Dode, L.; Vanoevelen, J.; van Baelen, K.; Parys, J.B.; Callewaert, G.; De Smedt, H.; Segaert, S.; Wuytack, F. SPCA1 pumps and Hailey-Hailey disease. Biochem. Biophys. Res. Commun 2004, 322, 1204–1213. [Google Scholar]
- Ramos-Castañeda, J.; Park, Y.N.; Liu, M.; Hauser, K.; Rudolph, H.; Shull, G.E.; Jonkman, M.F.; Mori, K.; Ikeda, S.; Ogawa, H.; et al. Deficiency of ATP2C1, a Golgi ion pump, induces secretory pathway defects in endoplasmic reticulum (ER)-associated degradation and sensitivity to ER stress. J. Biol. Chem 2005, 280, 9467–9473. [Google Scholar]
- Micaroni, M.; Perinetti, G.; Berrie, C.P.; Mironov, A.A. The SPCA1 Ca2+ pump and intracellular membrane trafficking. Traffic 2010, 11, 1315–1333. [Google Scholar]
- Raiko, L.; Siljamäki, E.; Mahoney, M.G.; Putaala, H.; Suominen, E.; Peltonen, J.; Peltonen, S. Hailey-Hailey disease and tight junctions: Claudins 1 and 4 are regulated by ATP2C1 gene encoding Ca(2+)/Mn(2+) ATPase SPCA1 in cultured keratinocytes. Exp. Dermatol 2010, 21, 586–591. [Google Scholar]
- Cockayne, S.E.; Rassl, D.M.; Thomas, S.E. Squamous cell carcinoma arising in Hailey-Hailey disease of the vulva. Br. J. Dermatol 2000, 142, 540–542. [Google Scholar]
- Holst, V.A.; Fair, K.P.; Wilson, B.G.; Patterson, J.W. Squamous cell carcinoma arising in Hailey-Hailey disease. J. Am. Acad. Dermatol 2000, 43, 368–371. [Google Scholar]
- Okunade, G.W.; Miller, M.L.; Azhar, M.; Andringa, A.; Sanford, L.P.; Doetschman, T.; Prasad, V.; Shull, G.E. Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J. Biol. Chem 2007, 282, 36517–26527. [Google Scholar]
- Tougeron, D.; Fauquembergue, E.; Rouquette, A.; Le Pessot, F.; Sesboüé, R.; Laurent, M.; Berthet, P.; Mauillon, J.; Di Fiore, F.; Sabourin, J.C.; et al. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Modern Pathol 2009, 22, 1186–1195. [Google Scholar]
- Morrissy, A.S.; Griffith, M.; Marra, M.A. Extensive relationship between antisense transcription and alternative splicing in the human genome. Genome Res 2011, 21, 1203–1212. [Google Scholar]
- Wootton, L.L.; Argent, C.C.; Wheatley, M.; Michelangeli, F. The expression, activity and localisation of the secretory pathway Ca2+-ATPase (SPCA1) in different mammalian tissues. Biochim. Biophys. Acta Biomembr 2004, 1664, 189–197. [Google Scholar]
- Baron, S.; Vangheluwe, P.; Sepúlveda, M.R.; Wuytack, F.; Raeymaekers, L.; Vanoevelen, J. The secretory pathway Ca(2+)-ATPase 1 is associated with cholesterol-rich microdomains of human colon adenocarcinoma cells. Biochim. Biophys. Acta Biomembr 2010, 1798, 1512–1521. [Google Scholar]
- Li, K.; Ramchandran, R. Natural antisense transcript: A concomitant engagement with protein-coding transcript. Oncotarget 2010, 1, 447–452. [Google Scholar]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar]
- Sepúlveda, M.R.; Vanoevelen, J.; Raeymaekers, L.; Mata, A.M.; Wuytack, F. Silencing the SPCA1 (secretory pathway Ca2+-ATPase isoform 1) impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J. Neurosci 2009, 29, 12174–12182. [Google Scholar]
- Chun, S.I.; Whang, K.C.; Su, W.P. Squamous cell carcinoma arising in Hailey-Hailey disease. J. Cutan. Pathol 1988, 5, 234–237. [Google Scholar]
- Manca, S.; Magrelli, A.; Cialfi, S.; Lefort, K.; Ambra, R.; Alimandi, M.; Biolcati, G.; Uccelletti, D.; Palleschi, C.; Screpanti, I.; et al. Oxidative stress activation of miR-125b is part of the molecular switch for Hailey-Hailey disease manifestation. Exp. Dermatol 2011, 20, 932–937. [Google Scholar]
- Feng, M.; Grice, D.M.; Faddy, H.M.; Nguyen, N.; Leitch, S.; Wang, Y.; Muend, S.; Kenny, P.A.; Sukumar, S.; Roberts-Thomson, S.J.; et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell 2010, 143, 84–98. [Google Scholar]
- Grice, D.M.; Vetter, I.; Faddy, H.M.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Golgi calcium pump secretory pathway calcium ATPase 1 (SPCA1) is a key regulator of insulin-like growth factor receptor (IGF1R) processing in the basal-like breast cancer cell line MDA-MB-231. J. Biol. Chem 2010, 285, 37458–37466. [Google Scholar]
- Colanzi, A.; Corda, D. Mitosis controls the Golgi and the Golgi controls mitosis. Curr. Opin. Cell Biol 2007, 19, 386–393. [Google Scholar]
- Von Blume, J.; Alleaume, A.M.; Cantero-Recasens, G.; Curwin, A.; Carreras-Sureda, A.; Zimmermann, T.; van Galen, J.; Wakana, Y.; Valverde, M.A.; Malhotra, V. ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev. Cell 2011, 20, 652–662. [Google Scholar]
- Lissandron, V.; Podini, P.; Pizzo, P.; Pozzan, T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc. Natl. Acad. Sci. USA 2010, 107, 9198–9203. [Google Scholar]
- Ton, V.K.; Mandal, D.; Vahadji, C.; Rao, R. Functional expression in yeast of the human secretory pathway Ca(2+), Mn(2+)-ATPase defective in Hailey-Hailey disease. J. Biol. Chem 2002, 277, 6422–6427. [Google Scholar]
- Durr, G.; Strayle, J.; Plemper, R.; Elbs, A.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar]
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Micaroni, M.; Malquori, L. Overlapping ATP2C1 and ASTE1 Genes in Human Genome: Implications for SPCA1 Expression? Int. J. Mol. Sci. 2013, 14, 674-683. https://doi.org/10.3390/ijms14010674
Micaroni M, Malquori L. Overlapping ATP2C1 and ASTE1 Genes in Human Genome: Implications for SPCA1 Expression? International Journal of Molecular Sciences. 2013; 14(1):674-683. https://doi.org/10.3390/ijms14010674
Chicago/Turabian StyleMicaroni, Massimo, and Lorenzo Malquori. 2013. "Overlapping ATP2C1 and ASTE1 Genes in Human Genome: Implications for SPCA1 Expression?" International Journal of Molecular Sciences 14, no. 1: 674-683. https://doi.org/10.3390/ijms14010674



