Population Subdivision of Japanese Flounder Paralichthys olivaceus in the Pacific Coast of Tohoku Japan Detected by Means of Mitochondrial Phylogenetic Information
Abstract
:1. Introduction
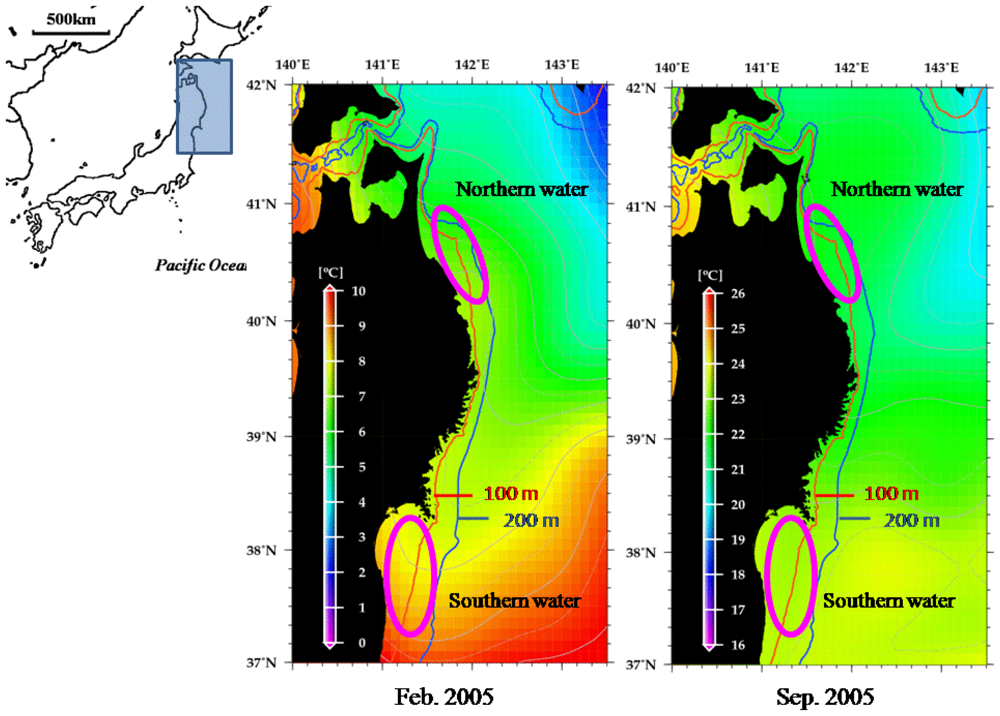
2. Results and Discussion
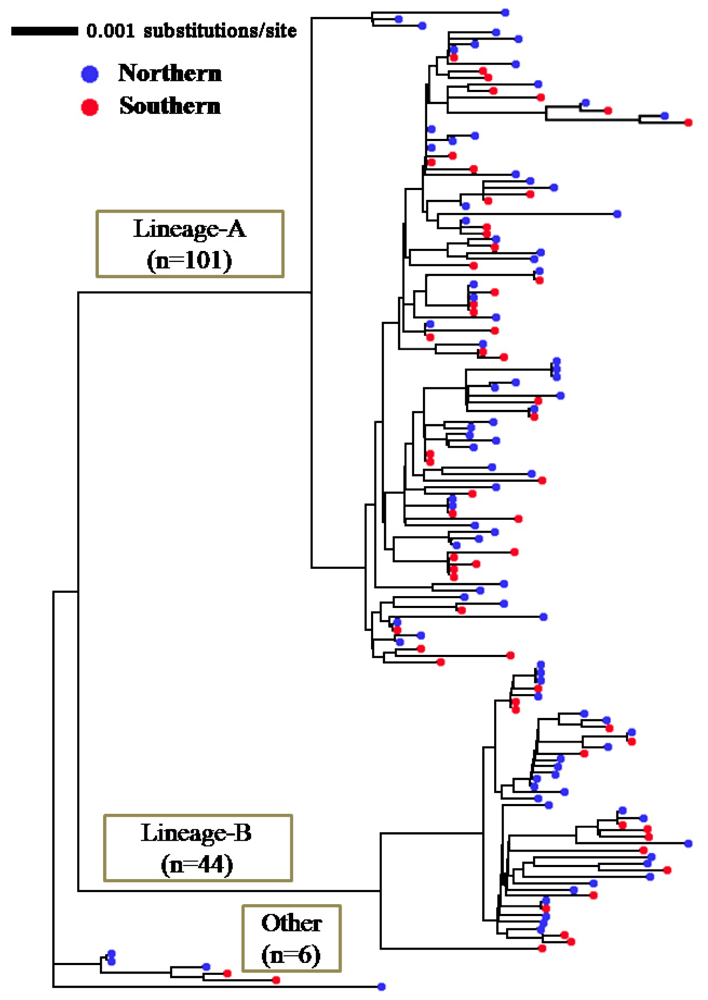
2.1. Genetic Variability of Japanese Flounder
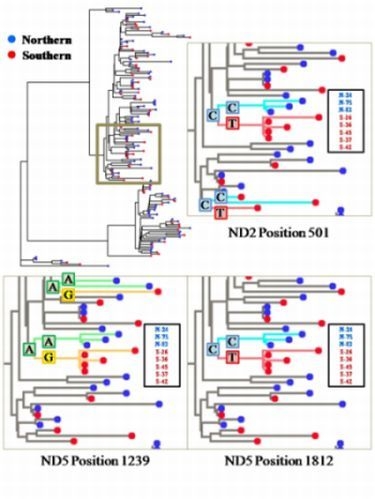
2.2. Segregation Sites, Character Mapping and F(ϕ)-Statistics
2.3. Discussion
3. Materials and Methods
3.1. Samples
3.2. Population Genetic Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestYutaka Kurita takes part in the Promotion Program for International Resources Survey from the Fisheries Agency of Japan in which fisheries stocks as management units are designated.
References
- Thorrold, S.M.; Jones, G.P.; Hellberg, M.E.; Burton, R.S.; Swearer, S.E.; Neoge, J.E.; Morgan, S.G.; Warner, R.R. Quantifying larval retention and connectivity in marine populations with artificial and natural markers. Bull. Mar. Sci 2002, 70, 291–308. [Google Scholar]
- Ishikawa, S.; Kimura, Y.; Tokai, T.; Tsukamoto, K.; Nishida, M. Genetic variation in the mitochondrial and nuclear DNA of Japanese conger Conger myriaster. Fish. Sci 2001, 67, 1081–1087. [Google Scholar]
- Schrey, A.W.; Heist, E.J. Microsatellite analysis of population structure in the shortfin mako (Isurusoxyrinchus). Can. J. Fish. Aquat. Sci 2003, 60, 670–675. [Google Scholar]
- De Innoceniis, S.; Lesti, A.; Livi, S.; Rossi, A.R.; Crosetti, D.; Sola, L. Microsatellite markers reveal population structure in gilthead sea bream Sparus auratus from the Atlantic Ocean and Mediterranean Sea. Fish. Sci 2004, 70, 852–859. [Google Scholar]
- Karaiskou, N.; Triantafyllidis, A.; Triantaphyllidis, C. Shallow genetic structure of three species of the genus Trachurus in European waters. Mar. Ecol. Prog. Ser 2004, 281, 193–205. [Google Scholar]
- Jeffrey, I.B.; Hale, P.; Degmam, B.M.; Degnan, S.M. Pleistocene isolation and recent gene flow in Haliotis asinine, an Indo-Pacific vetigastropod with limited dispersal capacity. Mol. Ecol 2007, 16, 289–304. [Google Scholar]
- Han, Z.Q.; Gao, T.X.; Yanagimoto, T.; Sakurai, Y. Genetic population structure of Nibea albiflora in Yellow Sea and East China Sea. Fish. Sci 2008, 74, 544–552. [Google Scholar]
- Shui, B.N.; Han, Z.Q.; Gao, T.X.; Miao, Z.Q.; Yanagimoto, T. Mitochondrial DNA variation in the East China Sea and Yellow Sea populations of Japanese Spanish Mackerel Scomberomorus niphonius. Fish. Sci 2009, 75, 593–600. [Google Scholar]
- Ward, R.D.; Woodwark, M.; Skibinski, D.O.F. A comparison of genetic diversity levels in marine, freshwater, and anadromous fishes. J. Fish Biol 1994, 44, 213–227. [Google Scholar]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Helberg, M.E. Gene flow and isolation among populations of marine animals. Ann. Rev. Ecol. Evol. Syst 2009, 40, 291–310. [Google Scholar]
- Swearer, S.E.; Shima, J.S.; Hellberg, M.E.; Thorrold, S.R.; Jones, G.P.; Robertson, D.R.; Morgan, S.G.; Selkoe, K.A.; Ruiz, G.M.; Warner, R.R. Evidence of self-recruitment in demersal marine populations. Bull. Mar. Sci 2002, 70, 251–271. [Google Scholar]
- Galarza, J.A.; Carreras-Carbonell, J.; Macpherson, E.; Pascual, M.; Roques, S.; Turner, G.F.; Rico, C. The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc. Natl. Acad. Sci. USA 2009, 106, 1473–1478. [Google Scholar]
- Nakashima, K.; Shindo, S. On the grouping of fishery grounds based on the species composition of the demersal fishes in the East China Sea and the Yellow Sea. Bull. Seikai. Reg. Fish. Res. Lab 1974, 44, 1–26. [Google Scholar]
- Minami, T. Life History. In Biology and Stock Enhancement of Japanese Flounder; Minami, T., Tanaka, M., Eds.; Koseisha-Koseikaku: Tokyo, Japan, 1997; pp. 9–24. [Google Scholar]
- Seikai, T.; Tanangonan, J.B.; Tanaka, M. Temperature influence on larval growth and metamorphosis of the Japanese flounder Paralichthys olivaceus in the laboratory. Bull. Japan. Soc. Sci. Fish 1986, 52, 977–982. [Google Scholar]
- Kinoshita, I.; Seikai, T.; Tanaka, M.; Kuwamura, K. Geographic variations in dorsal and anal ray counts of juvenile Japanese flounder, Paralichthys olivaceus, in the Japan Sea. Environ. Biol. Fish 2000, 57, 305–313. [Google Scholar]
- Nihira, A.; Takase, H.; Betsui, K.; Ishikawa, K. Results of mark-recapture experiments of flounder Paralichthys olivaceus (Temminck et Schlegel) on the coastal region of Ibaraki Prefecture. Bull. Ibaraki. Pref. Fish. Exp. Sta 1988, 26, 137–159. [Google Scholar]
- Ishito, Y. Distribution and migration of the young Japanese flounder (Paralichthys olivaceus) along the northeastern coast of Japan. Bull. Tohoku Natl. Fish. Res. Inst 1990, 52, 33–43. [Google Scholar]
- Tanaka, M.; Ohkawa, T.; Maeda, T.; Kinoshita, I.; Seikai, T.; Nishida, M. Ecological diversities and stock structure of the flounder in the Sea of Japan in relation to stock enhancement. Bull. Natl. Res. Inst. Aquacult 1997, 3, 77–85. [Google Scholar]
- Nakamura, R.; Watanabe, M.; Satoh, K. On the structure of subpopulation of Paralichthys olivaceus in the coastal area around Kantoh district, Japan. Bull. Kanagawa Pref. Fish. Res. Inst 2001, 6, 113–121. [Google Scholar]
- Yoneda, M.; Kurita, Y.; Kitagawa, D.; Ito, M. Spatial variation in the relationship between growth and maturation rate in male Japanese flounder Paralichthys olivaceus off the Pacific coast of northern Japan. J. Sea Res 2007, 57, 171–179. [Google Scholar]
- Yoneda, M.; Kurita, Y.; Kitagawa, D.; Ito, M.; Tomiyama, T.; Goto, T.; Takahashi, K. Age validation and growth variability of Japanese flounder Paralichthys olivaceus off the Pacific coast of northern Japan. Fish. Sci 2007, 73, 585–592. [Google Scholar]
- Kurita, Y.; Tamate, T.; Ito, M. Stock Assessment and Evaluation for Japanese Flounder (Fiscal Year 2010). In Marine Fisheries Stock Assessment and Evaluation for Japanese Waters (Fiscal Year 2010/2011); Fisheries Agency and Fisheries Research Agency of Japan: Tokyo, Japan, 2011; pp. 1358–1384. [Google Scholar]
- Fujii, T.; Nishida, M. High sequence variability in the mitochondrial DNA control region of the Japanese flounder Paralichthys olivaceus. Fish. Sci 1997, 63, 906–910. [Google Scholar]
- Sekino, M.; Hara, M. Application of microsatellite markers to population genetic studies of Japanese flounder Paralichthys olivaceus. Mar. Biotechnol 2001, 3, 572–589. [Google Scholar]
- Shigenobu, Y.; Hayashizaki, K.; Asahida, T.; Ida, H.; Saitoh, K. Stock structure of Japanese flounder inferred from morphological and genetic analyses. Fish. Sci 2007, 73, 1104–1112. [Google Scholar]
- Reynolds, R.W.; Smith, T.M.; Liu, C.; Chelton, D.B.; Casey, K.S.; Schlax, M.G. Daily high-resolution-blended analyses for sea surface temperature. J. Climate 2007, 20, 5473–5496. [Google Scholar]
- Meirmans, P.G.; Hedrick, P.W. Assessing population structure: Fst and related measures. Mol. Ecol. Resourc 2011, 11, 5–18. [Google Scholar]
- Kolbe, J.J.; Larson, A.; Losos, J.B.; de Queiroz, K. Admixture determines genetic diversity and population differentiation in the biological invasion of a lizard species. Biol. Lett 2008, 4, 434–437. [Google Scholar]
- Nielsen, E.E.; Hemmer-Hansen, J.; Larsen, P.F.; Bekkevold, D. Population genomics of marine fishes: identifying adaptive variation in space and time. Mol. Ecol 2009, 18, 3128–3150. [Google Scholar]
- Seikai, T. Mechanism of Abnormal Pigmentation. In Biology and Stock Enhancement of Japanese Flounder; Minami, T., Tanaka, M., Eds.; Koseisha-Koseikaku: Tokyo, Japan, 1997; pp. 63–73. [Google Scholar]
- Tomiyama, T.; Mizuno, T.; Watanabe, M.; Fujita, T.; Kawata, G. Patterns and frequency of hypermelanosis on the blind side in wild Japanese flounder. Nippon Suisan Gakkaishi 2008, 74, 171–176. [Google Scholar]
- Asahida, T.; Kobayashi, T.; Saitoh, K.; Nakayama, I. Tissue preservation and total DNA extraction from fish stored at ambient temperature using buffers containing high concentration of urea. Fish. Sci 1996, 62, 727–730. [Google Scholar]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol 2011, 28, 2731–2739. [Google Scholar]
- Swofford, D.L. Paup*: Phylogenetic Analysis Using Parsimony (and Other Methods), version 4; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar]
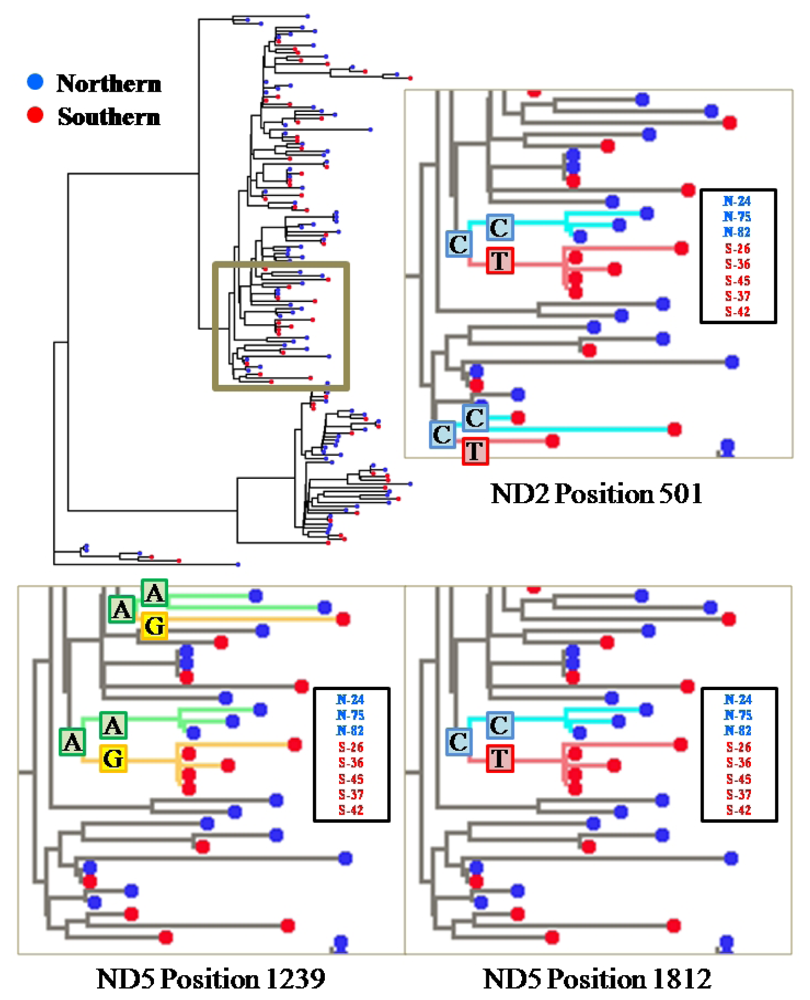
| Northern samples | Southern samples | p-value of Fisher’s exact-test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | G | C | T | A | G | C | T | ||
| ND2 Position 501 | 91 | 54 | 6 | 0.0034 | |||||
| ND5 Position 1239 | 91 | 54 | 6 | 0.0034 | |||||
| ND5 Position 1812 | 91 | 55 | 5 | 0.0089 | |||||
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shigenobu, Y.; Yoneda, M.; Kurita, Y.; Ambe, D.; Saitoh, K. Population Subdivision of Japanese Flounder Paralichthys olivaceus in the Pacific Coast of Tohoku Japan Detected by Means of Mitochondrial Phylogenetic Information. Int. J. Mol. Sci. 2013, 14, 954-963. https://doi.org/10.3390/ijms14010954
Shigenobu Y, Yoneda M, Kurita Y, Ambe D, Saitoh K. Population Subdivision of Japanese Flounder Paralichthys olivaceus in the Pacific Coast of Tohoku Japan Detected by Means of Mitochondrial Phylogenetic Information. International Journal of Molecular Sciences. 2013; 14(1):954-963. https://doi.org/10.3390/ijms14010954
Chicago/Turabian StyleShigenobu, Yuya, Michio Yoneda, Yutaka Kurita, Daisuke Ambe, and Kenji Saitoh. 2013. "Population Subdivision of Japanese Flounder Paralichthys olivaceus in the Pacific Coast of Tohoku Japan Detected by Means of Mitochondrial Phylogenetic Information" International Journal of Molecular Sciences 14, no. 1: 954-963. https://doi.org/10.3390/ijms14010954