Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus aureus Biofilms
Abstract
:1. Introduction
2. Results and Discussion
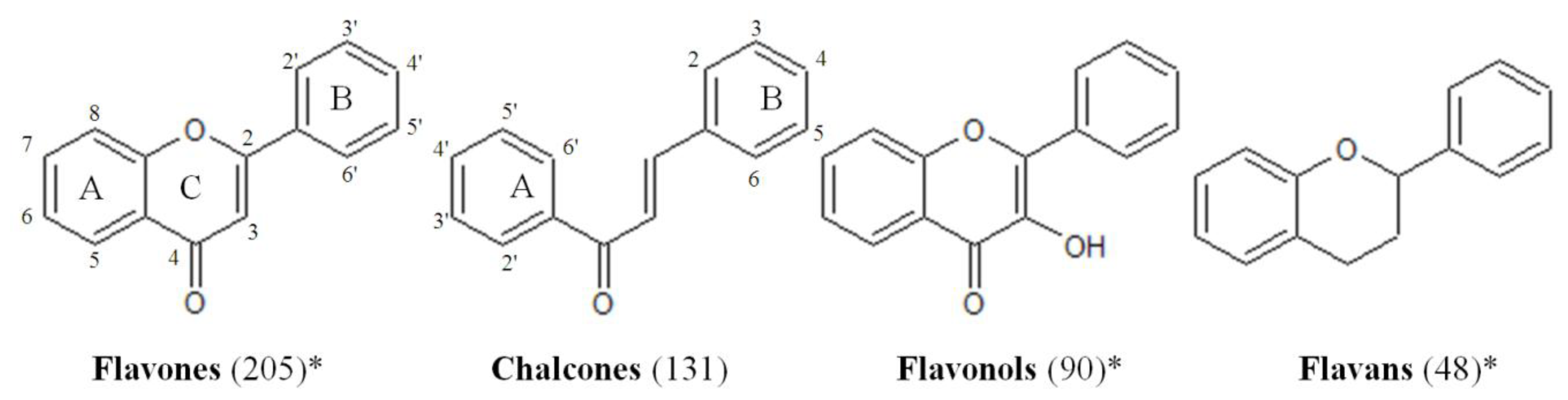
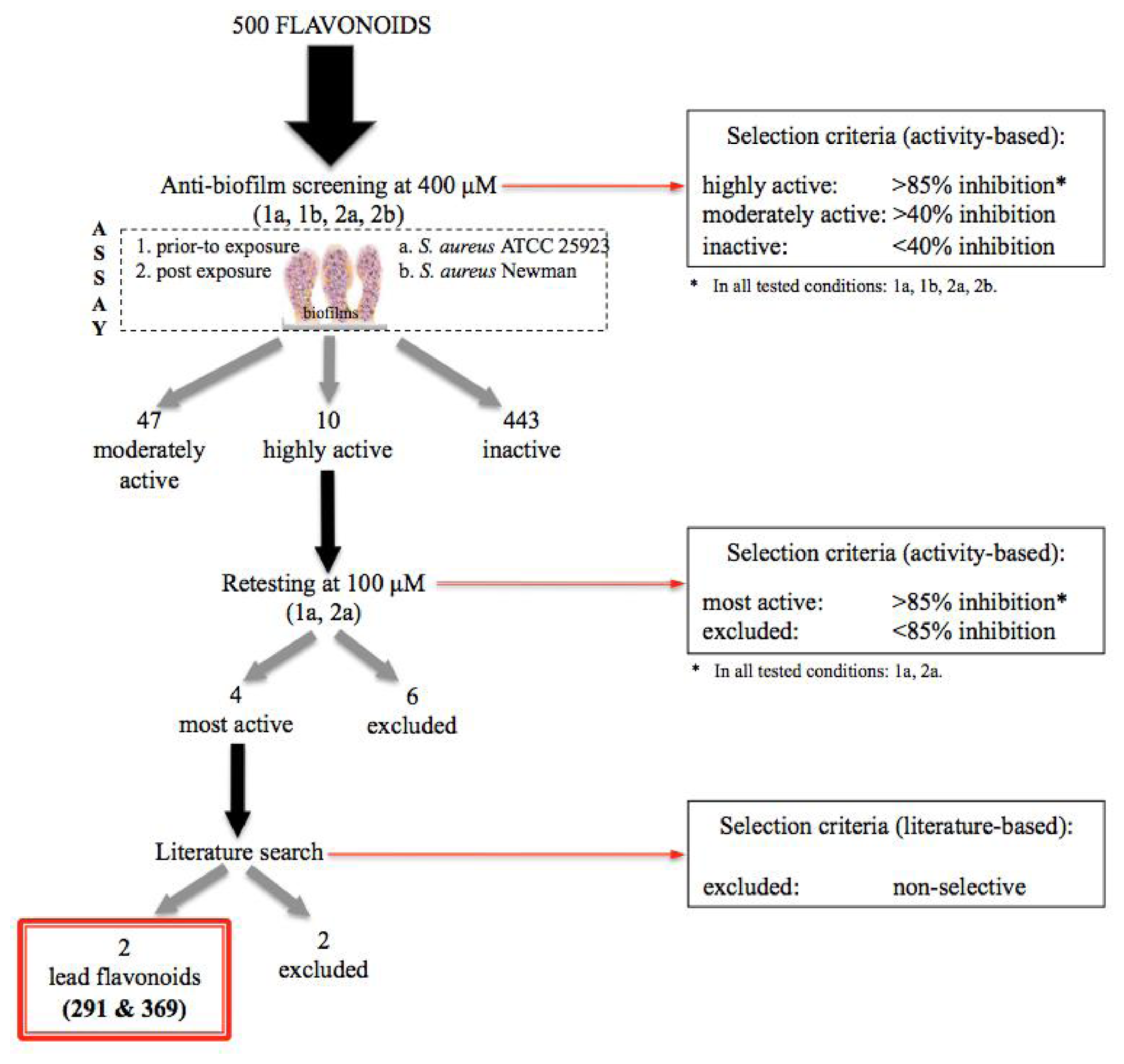
2.1. Design of the Flavonoid Anti-Biofilm Screening for More Meaningful Data Generation
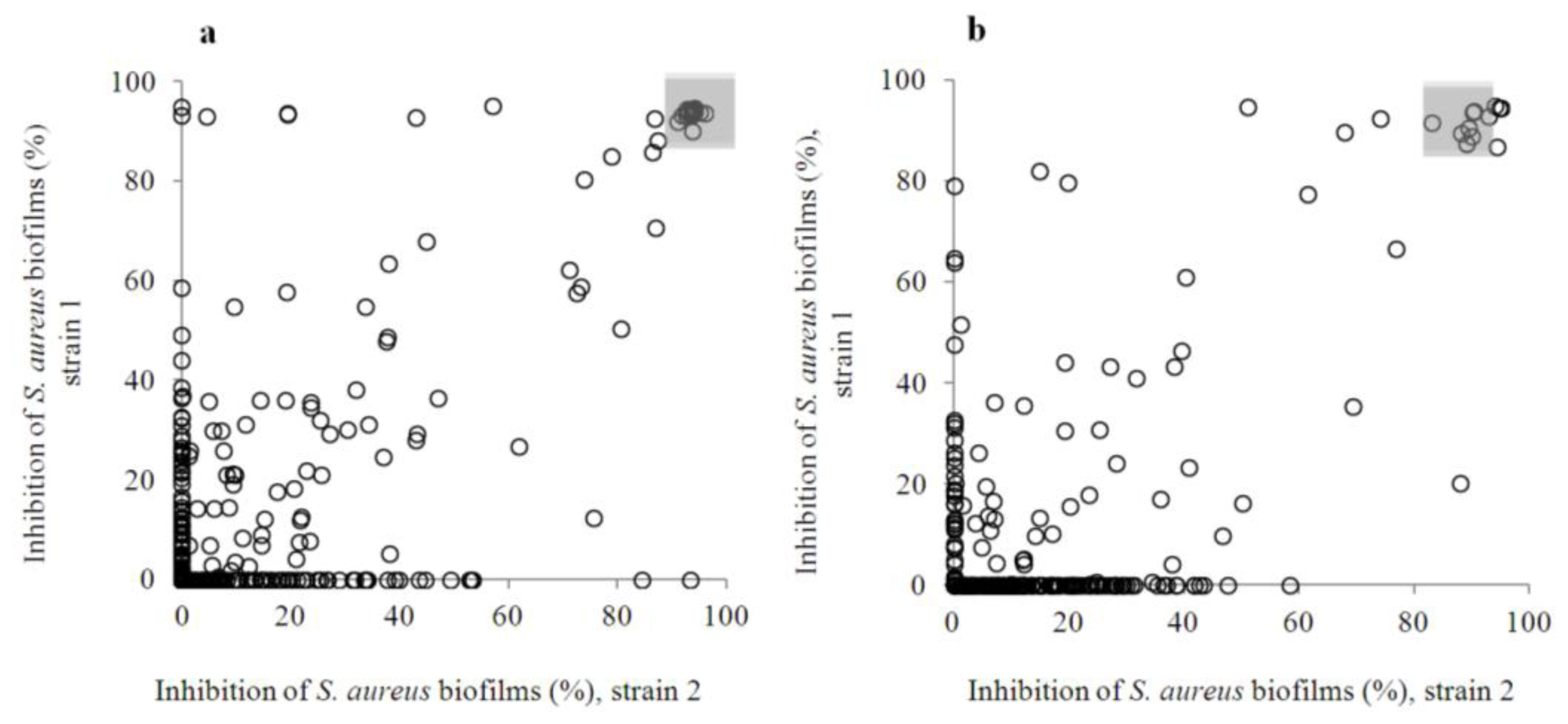
2.2. Anti-Biofilm Screening: Inactive and Moderately Active Flavonoids
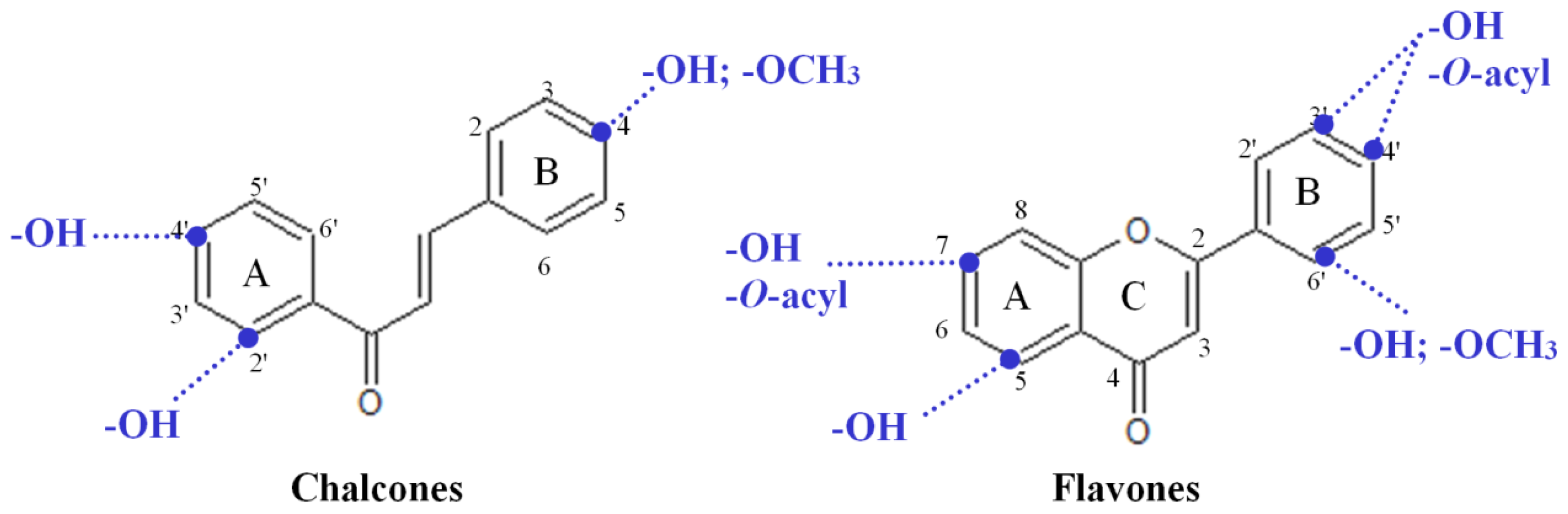
2.3. Highly Active Flavonoids and Further Selection Process
2.4. The Two Most Potent Flavonoids: Potencies, Efficacies and Mechanistic Insights
3. Experimental Section
3.1. Compounds Collection
3.2. Biofilm Assay
3.3. Exposure to Flavonoids
3.4. Quantification of Biofilms
3.5. Anti-Biofilm Potency and Efficacy Testing
3.6. Bacteriostatic and Bactericidal Effect on Planktonic Cells
3.7. Statistical Analysis and Data Processing
4. Conclusions
| Code | Company ID | Class | Inhibition of biofilm formation (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Primary screening at 400 μM | Reconfirmation trial at 100 μM | |||||||
| Prior-to-exposure | Post-exposure | Prior-to-exposure | Post-exposure | |||||
| strain 1 | strain 2 | strain 1 | strain 2 | strain 1 | ||||
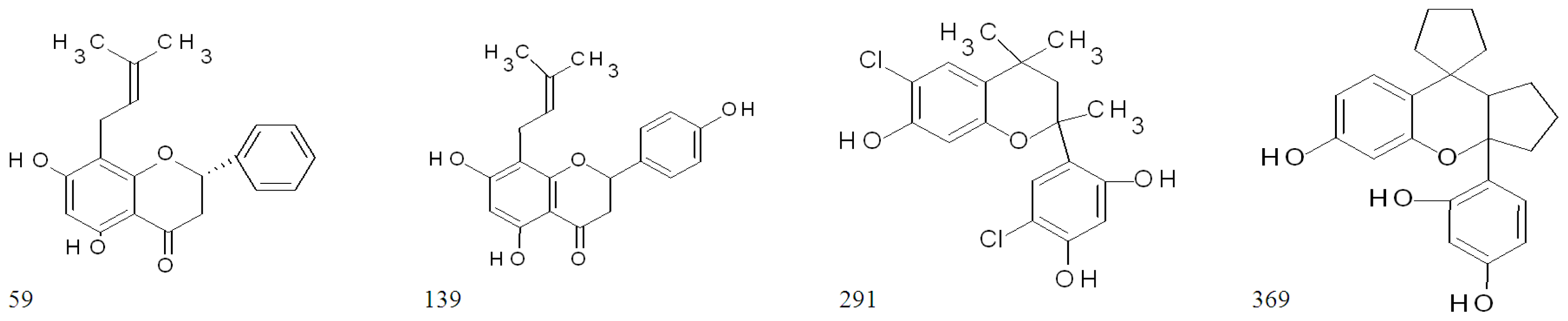
| 33 | ST014848 | isoflavone | 93.8 | 93.8 | 94.5 | 94.9 | 19.7 ± 5.6 | 0 |
| 59 | ST024709 | flavanone | 94.1 | 92.6 | 94.8 | 94.0 | 95.5 ± 0.1 | 92.5 ± 3.2 |
| 139 | ST056204 | flavanone | 93.8 | 95.1 | 86.9 | 94.3 | 95.0 ± 0.1 | 93.6 ± 1.1 |
| 291 | ST075672 | flavan | 93.2 | 92.6 | 94.6 | 94.7 | 95.4 ± 0.1 | 94.1 ± 1.2 |
| 369 | ST081006 | flavan | 93.6 | 96.1 | 93.9 | 90.3 | 95.3 ± 0.2 | 93.3 ± 1.7 |
| 424 | ST092293 | chalcone | 93.8 | 94.3 | 93.7 | 90.2 | 15.9 ± 5.1 | 0 |
| 432 | ST093738 | flavan | 94.1 | 94.0 | 89.4 | 88.1 | 0 | 0 |
| 446 | ST098360 | flavanone | 92.0 | 91.2 | 92.9 | 92.9 | 0 | 0 |
| 464 | ST095411 | chalcone | 93.7 | 93.7 | 90.5 | 89.4 | 0 | 0 |
| 469 | ST095417 | chalcone | 94.8 | 94.1 | 88.8 | 90.0 | 0 | 0 |
| Code | Effects on biofilms (IC50, μM) (μg/mL) (95% confidence intervals) | Effects on suspended bacteria | ||
|---|---|---|---|---|
| Prior-to-exposure | Post-exposure | MIC, μM | MBC, μM | |
| 291 | 10.2 (3.77) (8.9–11.6) | 27.9 (10.3) (22.9–33.9) | 20 (7.38) | 15 (5.54) |
| 369 | 17.7 (6.24) (12.7–24.8) | 60.5 (21.3) (49.6–73.9) | 40 (14.1) | 40 (14.1) |
| Penicillin G | 0.13 (0.048) (0.12–0.14) | 45.2%* | 0.12 (0.045) | 0.13 (0.048) |
| Code | Concentration (μM) | Log Reduction (biofilm phase) | Log Reduction (planktonic phase) |
|---|---|---|---|
| 291 | 20 | 0.6 | 0.1 |
| 80 | 1.5 | 0.7 | |
| 200 | 3.5 | 3.5 | |
| 400 | 4.6 | 4.7 | |
| 369 | 50 | 1.5 | 1.2 |
| 100 | 3.9 | 4.1 | |
| 250 | 3.1 | 9.0 | |
| 400 | 3.9 | 9.0 | |
| penicillin G | 400 | 1.0 | 4.0 |
Acknowledgments
Conflicts of Interest
References
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis 2001, 33, 1387–1392. [Google Scholar]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev 2002, 15, 167–193. [Google Scholar]
- Sauer, K.; Camper, A.K. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol 2001, 183, 6579–6589. [Google Scholar]
- Tote, K.; vanden Berghe, D.; Deschacht, M.; de Wit, K.; Maes, L.; Cos, P. Inhibitory efficacy of various antibiotics on matrix and viable mass of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Int. J. Antimicrob. Agents 2009, 33, 525–531. [Google Scholar]
- Landini, P.; Antoniani, D.; Burgess, J.G.; Nijland, R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl. Microbiol. Biotechnol 2010, 86, 813–823. [Google Scholar]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol 2003, 57, 677–701. [Google Scholar]
- Malic, S.; Hill, K.E.; Hayes, A.; Percival, S.L.; Thomas, D.W.; Williams, D.W. Detection and identification of specific bacteria in wound biofilms using peptide nucleic acid fluorescent in situ hybridization (PNA-FISH). Microbiology 2009, 155, 2603–2611. [Google Scholar]
- James, G.A.; Swogger, E.; Wolcott, R.; deLancey Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regener 2008, 16, 37–44. [Google Scholar]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202–211. [Google Scholar]
- Singh, P.K.; Schaefer, A.L.; Parsek, M.R.; Moninger, T.O.; Welsh, M.J.; Greenberg, E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000, 407, 762–764. [Google Scholar]
- Adair, C.G.; Gorman, S.P.; Feron, B.M.; Byers, L.M.; Jones, D.S.; Goldsmith, C.E.; Moore, J.E.; Kerr, J.R.; Curran, M.D.; Hogg, G.; et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med 1999, 25, 1072–1076. [Google Scholar]
- Worthington, R.J.; Richards, J.J.; Melander, C. Small molecule control of bacterial biofilms. Org. Biomol. Chem 2012, 10, 7457–7474. [Google Scholar]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Int. J. Pharm. Sci. Technol 2011, 6, 12–35. [Google Scholar]
- Kumar, B.; Sandhar, H.K.; Prasher, S.; Tiwari, P.; Salhan, M.; Sharma, P. A review of phytochemistry and pharmacology of flavonoids. Int. Pharm. Sci 2011, 1, 25–41. [Google Scholar]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther 2002, 96, 67–202. [Google Scholar]
- Alvesalo, J.; Vuorela, H.; Tammela, P.; Leinonen, M.; Saikku, P.; Vuorela, P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem. Pharmacol 2006, 71, 735–741. [Google Scholar]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol 2000, 56, 3–12. [Google Scholar]
- Sandberg, M.; Määttänen, A.; Peltonen, J.; Vuorela, P.M.; Fallarero, A. Automating a 96-well microtitre plate model for Staphylococcus aureus biofilms: An approach to screening of natural antimicrobial compounds. Int. J. Antimicrob. Agents 2008, 32, 233–240. [Google Scholar]
- Sandberg, M.E.; Schellmann, D.; Brunhofer, G.; Erker, T.; Busygin, I.; Leino, R.; Vuorela, P.M.; Fallarero, A. Pros and cons of using resazurin staining for quantification of viable Staphylococcus aureus biofilms in a screening assay. J. Microbiol. Methods 2009, 78, 104–106. [Google Scholar]
- Skogman, M.E.; Vuorela, P.M.; Fallarero, A. Combining biofilm matrix measurements with biomass and viability assays in susceptibility assessments of antimicrobials against Staphylococcus aureus biofilms. J. Antibiot 2012, 65, 453–459. [Google Scholar]
- Fallarero, A.; Skogman, M.; Kujala, J.; Rajaratnam, M.; Moreira, V.M.; Yli-Kauhaluoma, J.; Vuorela, P. (+)-Dehydroabietic acid, an abietane-type diterpene, inhibits Staphylococcus aureus biofilms in vitro. Int. J. Mol. Sci. 2013, 14, 12054–12072. [Google Scholar]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar]
- Mariscal, A.; Lopez-Gigosos, R.M.; Carnero-Varo, M.; Fernandez-Crehuet, J. Fluorescent assay based on resazurin for detection of activity of disinfectants against bacterial biofilm. Appl. Microbiol. Biotechnol 2009, 82, 773–783. [Google Scholar]
- Cushnie, T.P.; Hamilton, V.E.; Lamb, A.J. Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiol. Res 2003, 158, 281–289. [Google Scholar]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol 2010, 109, 515–527. [Google Scholar]
- Zeng, Z.; Qian, L.; Cao, L.; Tan, H.; Huang, Y.; Xue, X.; Shen, Y.; Zhou, S. Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol 2008, 79, 119–126. [Google Scholar]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol 2010, 76, 243–253. [Google Scholar]
- Zhao, X.; Mei, W.; Gong, M.; Zuo, W.; Bai, H.; Dai, H. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum. Molecules 2011, 16, 9775–9782. [Google Scholar]
- Feldman, M.; Santos, J.; Grenier, D. Comparative evaluation of two structurally related flavonoids, isoliquiritigenin and liquiritigenin, for their oral infection therapeutic potential. J. Nat. Prod 2011, 74, 1862–1867. [Google Scholar]
- Avila, H.P.; Smânia, E.F.A.; Monache, F.D.; Smânia, A. Structure-activity relationship of antibacterial chalcones. Bioorg. Med. Chem 2008, 16, 9790–9794. [Google Scholar]
- Alvarez, M.e.L.; Zarelli, V.E.; Pappano, N.B.; Debattista, N.B. Bacteriostatic action of synthetic polyhydroxylated chalcones against Escherichia coli. Biocell 2004, 28, 31–34. [Google Scholar]
- Li, H.Q.; Shi, L.; Li, Q.S.; Liu, P.G.; Luo, Y.; Zhao, J.; Zhu, H.L. Synthesis of C(7) modified chrysin derivatives designing to inhibit beta-ketoacyl-acyl carrier protein synthase III (FABH) as antibiotics. Bioorg. Med. Chem 2009, 17, 6264–6269. [Google Scholar]
- Lee, J.H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar]
- Pohjala, L.; Tammela, P. Aggregating behavior of phenolic compounds—A source of false bioassay results? Molecules 2012, 17, 10774–10790. [Google Scholar]
- McGovern, S.L.; Shoichet, B.K. Kinase inhibitors: Not just for kinases anymore. J. Med. Chem 2003, 46, 1478–1483. [Google Scholar]
- Dürig, A.; Kouskoumvekaki, I.; Vejborg, R.M.; Klemm, P. Chemoinformatics-assisted development of new anti-biofilm compounds. Appl. Microbiol. Biotechnol 2010, 87, 309–317. [Google Scholar]
- Fránová, J.; Pavlík, M. Testing of antidiabetic and antioxidative effect of the flavonoid osajin in an experiment. Ceska Slov. Farm 2007, 56, 200–204. [Google Scholar]
- Suksamrarn, A.; Chotipong, A.; Suavansri, T.; Boongird, S.; Timsuksai, P.; Vimuttipong, S.; Chuaynugul, A. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch. Pharm. Res 2004, 27, 507–511. [Google Scholar]
- Sufian, A.S.; Ramasamy, K.; Ahmat, N.; Zakaria, Z.A.; Yusof, M.I. Isolation and identification of antibacterial and cytotoxic compounds from the leaves of Muntingia calabura L. J. Ethnopharmacol 2013, 146, 198–204. [Google Scholar]
- Sánchez, I.; Gómez-Garibay, F.; Taboada, J.; Ruiz, B.H. Antiviral effect of flavonoids on the dengue virus. Phytother. Res 2000, 14, 89–92. [Google Scholar]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol 2008, 116, 383–396. [Google Scholar]
- Mizobuchi, S.; Sato, Y. Antifungal activities of hop bitter resins and related compounds. Agric. Biol. Chem 1985, 49, 399–403. [Google Scholar]
- Shen, G.; Huhman, D.; Lei, Z.; Snyder, J.; Sumner, L.W.; Dixon, R.A. Characterization of an isoflavonoid-specific prenyltransferase from Lupinus albus. Plant Physiol 2012, 159, 70–80. [Google Scholar]
- Chang, S.Y.; Cheng, M.J.; Peng, C.F.; Chang, H.S.; Chen, I.S. Antimycobacterial butanolides from the root of Lindera akoensis. Chem. Biodiversity 2008, 5, 2690–2698. [Google Scholar]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol 2005, 100, 80–84. [Google Scholar]
- Pitts, B.; Hamilton, M.A.; Zelver, N.; Stewart, P.S. A microtiter-plate screening method for biofilm disinfection and removal. J. Microbiol. Methods 2003, 54, 269–276. [Google Scholar]
- Jensen, P.; Givskov, M.; Bjarnsholt, T.; Moser, C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol 2010, 59, 292–305. [Google Scholar]
- Müller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother 2008, 61, 1281–1287. [Google Scholar]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screening 1999, 4, 67–73. [Google Scholar]
- Bollini, S.; Herbst, J.J.; Gaughan, G.T.; Verdoorn, T.A.; Ditta, J.; Dubowchik, G.M.; Vinitsky, A. High-throughput fluorescence polarization method for identification of fkbp12 ligands. J. Biomol. Screening 2002, 7, 526–530. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus aureus Biofilms. Int. J. Mol. Sci. 2013, 14, 19434-19451. https://doi.org/10.3390/ijms141019434
Manner S, Skogman M, Goeres D, Vuorela P, Fallarero A. Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus aureus Biofilms. International Journal of Molecular Sciences. 2013; 14(10):19434-19451. https://doi.org/10.3390/ijms141019434
Chicago/Turabian StyleManner, Suvi, Malena Skogman, Darla Goeres, Pia Vuorela, and Adyary Fallarero. 2013. "Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus aureus Biofilms" International Journal of Molecular Sciences 14, no. 10: 19434-19451. https://doi.org/10.3390/ijms141019434




