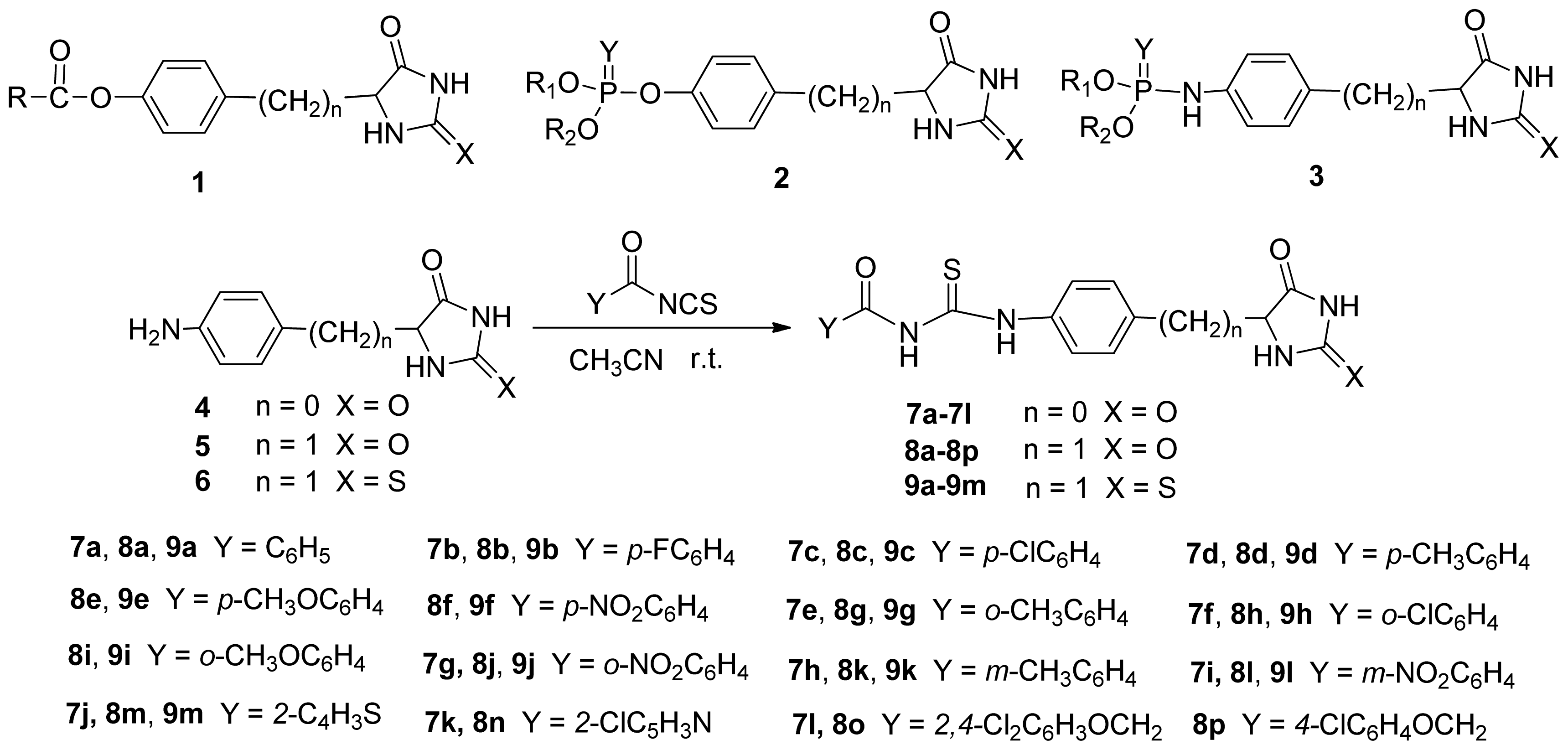
3.2.2. General Procedure for the Synthesis of Compounds 7, 8 and 9
To a stirred solution of 20 mmol 5-(4-aminophenyl)-hydantoin (4), or 5-(4-aminobenzyl)-hydantoin (5) or 5-(4-aminobenzyl)-2-thiohydantoin (6) in 20 mL of anhydrous acetonitrile, the acyl isothiocyanate solution in acetonitrile freshly prepared were added dropwise at ambient temperature. The reaction was monitored by TLC. After leaving it overnight, the reaction was stopped and the product was filtered. The products were further purified by recrystallization using DMF-EtOH-H2O to afford the compounds 7, 8 and 9.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)benzamide 7a, white solid, yield 82%, m.p. 140–142 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.60 (s, 1H, NH), 11.58 (s, 1H, NH), 10.82 (s, 1H, NH), 8.43 (s, 1H, NH), 7.97 (d, J = 8.4 Hz, 2H, ArH), 7.73–7.52 (m, 5H, ArH), 7.38 (d, J = 8.4 Hz, 2H, ArH), 5.20 (s, 1H, CH); IR (KBr) ν: 3160, 3053, 1787, 1725, 1672, 1597 cm−1. Anal calcd. for C17H14N4O3S: C 57.62, H 3.98, N 15.81; Found: C 57.59, H 3.90, N 15.78.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-4-fluorobenzamide 7b, white solid, yield 70%, m.p. 146–148 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.55 (s, 1H, NH), 11.64 (s, 1H, NH), 10.83 (s, 1H, NH), 8.44 (s, 1H, NH), 8.09–8.03 (m, 2H, ArH), 7.70 (d, 2H, J = 8.4 Hz, ArH), 7.42–7.32 (m, 4H, ArH), 5.20 (s, 1H, CH); IR (KBr) ν: 3125, 3041, 1784, 1729, 1666, 1601 cm−1. Anal calcd. for C17H13FN4O3S: C 54.83, H 3.52, N 15.05; Found: C 54.80, H 3.50, N 15.01.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-4-chlorobenzamide 7c, white solid, yield 55%, m.p.230–232 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.50 (s, 1H, NH), 11.69 (s, 1H, NH), 10.81 (s, 1H, NH), 8.43 (s, 1H, NH), 7.99 (d, J = 7.4 Hz, 2H, ArH), 7.70 (d, J = 8.4 Hz, 2H, ArH), 7.62 (d, J = 7.4 Hz, 2H, ArH), 7.37 (d, J = 8.4 Hz, 2H, ArH), 5.20 (s, 1H, CH); IR (KBr) ν: 3145, 3035, 1783, 1728, 1670, 1593 cm−1; Anal calcd. for C17H13ClN4O3S: C 52.51, H 3.37, N 14.41; Found: C 52.53, H 3.41, N 14.36.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-4-methylbenzamide 7d, white solid, yield 53%, m.p.220–222 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.65 (s, 1H, NH), 11.50 (s, 1H, NH), 10.82 (s, 1H, NH), 8.44 (s, 1H, NH), 7.91 (d, J = 7.5 Hz, 2H, ArH), 7.71 (d, J = 8.4 Hz, 2H, ArH), 7.39–7.34 (m, 4H, ArH), 5.20 (s, 1H, CH), 2.40 (s, 3H, CH3); IR (KBr) ν: 3150, 3035, 1786, 1726, 1664, 1598 cm−1; Anal calcd. for C18H16N4O3S: C 58.68, H 4.38, N 15.21; Found: C 58.63, H 4.40, N 15.11.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-2-methylbenzamide 7e, white solid, yield 54%, m.p.138–140 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.62 (s, 1H, NH), 11.51 (s, 1H, NH), 10.82 (s, 1H, NH), 8.43 (s, 1H, NH), 7.84–7.70 (m, 4H, ArH), 7.47–7.37 (m, 4H, ArH), 5.20 (s, 1H, CH), 2.40 (s, 3H, CH3); IR (KBr) ν: 3170, 3045, 1789, 1715, 1677, 1598 cm−1; Anal calcd. for C18H16N4O3S: C 58.68, H 4.38, N 15.21; Found: C 58.62, H 4.55, N 15.35.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-2-chlorobenzamide 7f, white solid, yield 55%, m.p.222–224 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.34 (s, 1H, NH), 12.03 (s, 1H, NH), 10.83 (s, 1H, NH), 8.44 (s, 1H, NH), 7.72 (d, J = 7.4 Hz, 2H, ArH), 7.65–7.43 (m, 4H, ArH), 7.38 (d, J = 7.4 Hz, 2H, ArH), 5.21 (s, 1H, CH); IR (KBr) ν: 3301, 3056, 1783, 1715, 1694, 1593 cm−1; Anal calcd. for C17H13ClN4O3S: C 52.51, H 3.37, N 14.41; Found: C 52.60, H 3.41, N 14.44.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-2-nitrobenzamide 7g, white solid, yield 63%, m.p.234–236 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.25 (s, 1H, NH), 12.17 (s, 1H, NH), 10.84 (s, 1H, NH), 8.46 (s, 1H, NH), 8.23 (d, J = 8.1 Hz, 1H, ArH), 7.94–7.71 (m, 5H, ArH), 7.39 (d, J = 7.4 Hz, 2H, ArH), 5.22 (s, 1H, CH); IR (KBr) ν: 3146, 3034, 1764, 1716, 1690, 1593 cm−1; Anal calcd. for C17H13N5O5S: C 51.12, H 3.28, N 17.54; Found: C 51.14, H 3.21, N 17.25.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-3-methylbenzamide 7h, white solid, yield 54%, m.p.158–160 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.62 (s, 1H, NH), 11.54 (s, 1H, NH), 10.83 (s, 1H, NH), 8.45 (s, 1H, NH), 7.83–7.70 (m, 4H, ArH), 7.49–7.36 (m, 4H, ArH), 5.21 (s, 1H, CH), 2.39 (s, 3H, CH3); IR (KBr) ν: 3210, 3047, 1769, 1726, 1668, 1600 cm−1; Anal calcd. for C18H16N4O3S: C 58.68, H 4.38, N 15.21; Found: C 58.64, H 4.40, N 15.16.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)-3-nitrobenzamide 7i, white solid, yield 52%, m.p.162–164 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.42 (s, 1H, NH), 12.04 (s, 1H, NH), 10.83 (s, 1H, NH), 8.78 (d, J = 2.1 Hz, 1H, ArH), 8.51–8.47 (m, 1H, ArH), 8.45 (s, 1H, NH), 8.37 (dd, J = 7.8, 2.4Hz, 1H, ArH), 7.84 (t, J = 7.8 Hz, 1H, ArH), 7.72 (d, J = 8.4 Hz, 2H, ArH), 7.39 (d, J = 8.4 Hz, 2H, ArH), 5.21 (s, 1H, CH); IR (KBr) ν: 3229, 3047, 1776, 1723, 1677, 1600 cm−1; Anal calcd. for C17H13N5O5S: C 51.12, H 3.28, N 17.54; Found: C 51.02, H 3.30, N 17.54.
N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)thiophene-2-carboxamide 7j, white solid, yield 80%, m.p. 226–228 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.44 (s, 1H, NH), 11.64 (s, 1H, NH), 10.81 (s, 1H, NH), 8.43 (s, 1H, NH), 8.38 (d, J = 3.8 Hz, 1H, ThH), 8.05 (d, J = 5.0 Hz, 1H, ThH), 7.70 (d, J = 7.4 Hz, 2H, ArH), 7.36 (d, J = 7.4 Hz, 2H, ArH), 7.26 (dd, J = 3.8, 5.0 Hz, 1H, ThH), 5.20 (s, 1H, CH); IR (KBr) ν: 3138, 3043, 1778, 1722, 1657, 1592 cm−1. Anal calcd. for C15H12N4O3S2: C 49.99, H 3.36, N 15.55; Found: C 49.97, H 3.39, N 15.56.
2-Chloro-N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)isonicotinamide 7k, white solid, yield 73%, m.p. 212–214 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.25 (s, 1H, NH), 11.96 (s, 1H, NH), 10.81 (s, 1H, NH), 8.62 (d, J = 5.4 Hz, 1H, PyH), 8.43 (s, 1H, NH), 7.99 (s, 1H, PyH), 7.84 (dd, J = 1.2, 5.4 Hz, 1H, PyH), 7.70 (d, J = 7.4 Hz, 2H, ArH), 7.39 (d, J = 7.4 Hz, 2H, ArH), 5.20 (s, 1H, CH); IR (KBr) ν: 3150, 3042, 1765, 1719, 1666, 1593 cm−1. Anal calcd. for C16H12ClN5O3S: C 49.30, H 3.10, N 17.97; Found: C 49.26, H 3.08, N 17.90.
2-(2,4-dichlorophenoxy)-N-((4-(2,4-dioxoimidazolidin-5-yl)phenyl)carbamothioyl)acetamide 7l, white solid, yield 59%, m.p. 258–260 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 11.85 (s, 1H, NH), 10.77 (s, 1H, NH), 10.24 (s, 1H, NH), 8.37 (s, 1H, NH), 7.63–7.60 (m, 3H, ArH), 7.37 (dd, J = 2.4, 8.4 Hz, 1H, ArH), 7.27 (d, J = 7.5 Hz, 2H, ArH), 7.09 (d, J = 8.4 Hz, 1H, ArH), 5.11 (s, 1H, CH), 4.86 (s, 2H, CH2); IR (KBr) ν: 3288, 3060, 1780, 1740, 1682, 1600 cm−1. Anal calcd. for C18H14Cl2N4O4S: C 47.69, H 3.11, N 12.36; Found: C 47.58, H 3.12, N 12.34.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)benzamide 8a, white solid, yield 93%, m.p. 240–242 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.61 (s, 1H, NH), 11.53 (s, 1H, NH), 10.48 (s, 1H, NH), 7.99–7.96 (m, 3H, ArH+NH), 7.70–7.64 (m, 3H, ArH), 7.57–7.52 (m, 2H, ArH),7.23 (d, J = 7.8 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.99–2.89 (m, 2H, CH2); IR (KBr) ν: 3172, 3064, 1766, 1726, 1668, 1597 cm−1. Anal calcd. for C18H16N4O3S: C 58.68, H 4.38, N 15.21; Found: C 58.98, H 4.30, N 15.25.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-flurobenzamide 8b, white solid, yield 83%, m.p. 216–218 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.56 (s, 1H, NH), 11.58 (s, 1H, NH), 10.48(s, 1H, NH), 8.09–8.03(m, 2H, ArH), 7.96 (s, 1H, NH), 7.64 (d, J = 8.0 Hz, 2H, ArH), 7.40–7.35 (m, 2H, ArH), 7.23 (d, J = 8.0 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH,), 2.97–2.94 (m, 2H, CH2); IR (KBr) ν: 3178, 3060, 1762, 1727, 1669, 1600 cm−1. Anal calcd for C18H15FN4O3S: C 55.95, H 3.91, N 14.50; Found: C 55.86, H 4.01, N 14.51.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-chlorobenzamide 8c, white solid, yield 63%, m.p. 232–234 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.51 (s, 1H, NH), 11.64 (s, 1H, NH), 10.49 (s, 1H, NH), 7.98–7.93 (m, 3H, ArH + NH), 7.66–7.56 (m, 4H, ArH), 7.23 (d, 2H, J = 8.4 Hz, ArH), 4.37–4.33 (m, 1H, CH), 2.95–2.93 (m, 2H, CH2); IR (KBr) ν: 3155, 3034, 1758, 1726, 1666, 1594 cm−1. Anal calcd. for C18H15ClN4O3S: C 53.67, H 3.75, N 13.91; Found: C 53.78, H 3.79, N 13.90.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-methylbenzamide 8d, white solid, yield 66%, m.p. 228–230 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.67 (s, 1H, NH), 11.43 (s, 1H, NH), 10.48 (s, 1H, NH), 7.96 (s, 1H, NH), 7.91 (d, J = 8.1 Hz, 2H, ArH), 7.65 (d, J = 8.4 Hz, 2H, ArH), 7.35 (d, J = 8.1 Hz, 2H, ArH), 7.23 (d, J = 8.4 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.96–2.93 (m, 2H, CH2), 2.37 (s, 3H, CH3); IR (KBr) ν: 3171, 3067, 1767, 1730, 1668, 1598 cm−1. Anal calcd. for C19H18N4O3S: C 59.67, H 4.74, N 14.65; Found: C 59.58, H 4.67, N 14.48.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-methoxybenzamide 8e, white solid, yield 69%, m.p. 224–226 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.74 (s, 1H, NH), 11.39 (s, 1H, NH), 10.50 (s, 1H, NH), 8.02 (d, J = 8.7 Hz, 2H, ArH,), 7.99 (s, 1H, NH),7.64 (d, J = 8.4 Hz, 2H, ArH), 7.22 (d, J = 8.4 Hz, 2H, ArH), 7.07 (d, J = 8.7 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 3.86 (s, 3H, OCH3), 2.95–2.93 (m, 2H, CH2); IR (KBr) ν: 3176, 3051, 1766, 1726, 1667, 1596 cm−1. Anal calcd. for C19H18N4O4S: C 57.27, H 4.55, N 14.16; Found: C 57.22, H 4.55, N 14.03.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-nitrobenzamide 8f, yellow solid, yield 82%, m.p. 226–228 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.75 (s, 1H, NH), 11.47(s, 1H, NH), 10.44 (s, 1H, NH), 8.38–7.93 (m, 5H, ArH + NH), 7.35–7.20 (m, 4H, ArH), 4.38–4.33 (m, 1H, CH), 2.97–2.92 (m, 2H, CH2); IR (KBr) ν: 3112, 3047, 1768, 1728, 1667, 1592 cm−1. Anal calcd. for C18H15N5O5S: C 52.30, H 3.66, N 16.94; Found: C 52.23, H 3.68, N 16.85.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-methylbenzamide 8g, white solid, yield 56%, m.p. 212–214 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.56 (s, 1H, NH), 11.68 (s, 1H, NH), 10.48 (s, 1H, NH), 7.96 (s, 1H, NH), 7.67 (d, J = 8.4 Hz, 2H, ArH), 7.51~7.22 (m, 6H, ArH), 4.37–4.34 (m, 1H, CH), 2.96–2.91 (m, 2H, CH2), 2.42 (s, 3H, CH3); IR (KBr) ν: 3181, 3066, 1767, 1722, 1673, 1595 cm−1. Anal calcd. for C19H18N4O3S: C 59.67, H 4.74, N 14.65; Found: C 59.69, H 4.75, N 14.74.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-chlorobenzamide 8h, white solid, yield 60%, m.p. 216–218 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.36 (s, 1H, NH), 11.98 (s, 1H, NH), 10.49 (s, 1H, NH), 7.97 (s, 1H, NH), 7.67–7.43 (m, 6H, ArH), 7.23 (d, J = 8.5 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.99–2.93 (m, 2H, CH2); IR (KBr) ν: 3194, 3060, 1766, 1717, 1679, 1592, 1537 cm−1. Anal calcd. for C18H15ClN4O3S: C 53.67, H 3.75, N 13.91; Found: C 53.60, H 3.75, N 13.86.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-methoxybenzamide 8i, white solid, yield 60%, m.p. 216–218 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.57 (s 1H, NH), 12.21 (s, 1H, NH), 10.49 (s, 1H, NH), 7.97 (s, 1H, NH), 7.94–7.91 (m, 1H, ArH), 7.70–7.64 (m, 3H, ArH), 7.31–7.14 (m, 4H, ArH), 4.37–4.34 (m, 1H, CH), 4.01 (s, 3H, OCH3), 2.96–2.94 (m, 2H, CH2); IR (KBr) ν: 3219, 3036, 1769, 1716, 1665, 1595 cm−1. Anal calcd. for C19H18N4O4S: C 57.27, H 4.55, N 14.16; Found: C 57.51, H 4.53, N 14.14.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-nitrobenzamide 8j, yellow solid, yield 50%, m.p. 222–224 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.27 (s, 1H, NH), 12.12 (s, 1H, NH), 10.49 (s, 1H, NH), 8.24–8.21 (m, 1H, ArH), 7.96 (s, 1H, NH), 7.94–7.77 (m, 3H, ArH), 7.66 (d, 2H, J = 8.4 Hz, ArH), 7.24 (d, 2H, J = 8.4 Hz, ArH), 4.38–4.34 (m, 1H, CH), 2.97–2.95 (m, 2H, CH2); IR (KBr) ν: 3219, 3036, 1769, 1716, 1665, 1595 cm−1. Anal calcd. for C18H15N5O5S: C 52.30, H 3.66, N 16.94; Found: C 52.25, H 3.76, N 16.84.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-3-methylbenzamide 8k, white solid, yield 64%, m.p. 220–221 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.64 (s, 1H, NH), 11.48 (s, 1H, NH), 10.49 (s, 1H, NH), 7.97 (s, 1H, NH), 7.83–7.63 (m, 4H, ArH), 7.49–7.40 (m, 2H, ArH), 7.23 (d, J = 8.4 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.97–2.92 (m, 2H, CH2), 2.36 (s, 3H, CH3); IR (KBr) ν: 3169, 3056, 1767, 1726, 1668, 1596 cm−1. Anal calcd. for C19H18N4O3S: C 59.67, H 4.74, N 14.65; Found: C 59.62, H 4.75, N 14.54.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-3-nitrobenzamide 8l, little yellow solid, yield 79%, m.p. 168–170 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.43 (s, 1H, NH), 12.01 (s, 1H, NH), 10.49 (s, 1H, NH), 8.78 (s, 1H, ArH), 8.51–8.36 (m, 2H, ArH), 7.99 (s, 1H, NH), 7.86–7.68 (m, 3H, ArH), 7.24 (d, J = 8.4 Hz, 2H, ArH), 4.38–4.34 (m, 1H, CH), 2.97–2.91 (m, 2H, CH2); IR (KBr) ν: 3180, 3056, 1770, 1719, 1669, 1602 cm−1. Anal calcd. for C18H15N5O5S: C 52.30, H 3.66, N 16.94; Found: C 52.32, H 3.60, N 16.84.
N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)thiophene-2-carboxamide 8m, white solid, yield 82%, m.p. 244–246 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.46 (s, 1H, NH), 11.58 (s, 1H, NH), 10.48 (s, 1H, NH), 8.39–8.37 (m, 1H, ThH), 8.06–8.04 (m, 1H, ThH), 7.96 (s, 1H, NH), 7.63 (d, J = 8.5 Hz, 2H, ArH), 7.27–7.21 (m, 3H, ArH + ThH), 4.37–4.32 (m, 1H, CH), 2.96–2.91 (m, 2H, CH2); IR (KBr) ν: 3171, 3061, 1762, 1716, 1654, 1596 cm−1. Anal calcd. for C16H14N4O3S2: C 51.32, H 3.77, N 14.96; Found: C 51.60, H 3.81, N 14.96.
2-Chloro-N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)isonicotinamide 8n, white solid, yield 80%, m.p. 202–204 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.25 (s, 1H, NH), 11.92 (s, 1H, NH), 10.48 (s, 1H, NH), 8.61 (d, J = 5.1 Hz, 1H, PyH), 7.99–7.82 (m, 3H, PyH + NH), 7.63 (d, J = 8.3 Hz, 2H, ArH), 7.24 (d, J = 8.3 Hz, 2H, ArH), 4.37–4.34 (m, 1H, CH), 2.99–2.89 (m, 2H, CH2); IR (KBr) ν: 3150, 3042, 1765, 1719, 1666, 1593 cm−1. Anal calcd. for C17H14ClN5O3S: C 50.56, H 3.49, N 17.34; Found: C 50.70, H 3.57, N 17.27.
2-(2,4-dichlorophenoxy)-N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)acetamide 8o, white solid, yield 71%, m.p. 262–264 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 11.95 (s, 1H, NH), 10.41 (s, 1H, NH), 10.14 (s, 1H, NH), 7.91 (s, 1H, NH), 7.50 (d, J = 8.5 Hz, 2H, ArH), 7.40~7.08 (m, 4H, ArH), 4.83 (s, 2H, CH2), 4.36–4.30 (m, 1H, CH), 2.90–2.81 (m, 2H, CH2); IR (KBr) ν: 3159, 3048, 1762, 1732, 1669, 1597 cm−1. Anal calcd. for C19H16Cl2N4O4S: C 48.83, H 3.45, N 11.99; Found: C 48.72, H 3.55, N 11.92.
2-(4-dichlorophenoxy)-N-((4-((2,4-dioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)acetamide 8p, white solid, yield 70%, m.p. 210–212 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 11.94 (s, 1H, NH), 10.42 (s, 1H, NH), 10.06 (s, 1H, NH), 7.92 (s, 1H, NH), 7.52 (d, J = 8.5 Hz, 2H, ArH), 7.39~7.33 (m, 2H, ArH), 7.14~7.10 (m, 2H, ArH), 7.04~6.99 (m, 2H, ArH), 4.69 (s, 2H, CH2), 4.34–4.28 (m, 1H, CH), 2.93–2.85 (m, 2H, CH2); IR (KBr) ν: 3116, 3043, 1769, 1709, 1687, 1598 cm−1. Anal calcd. for C19H17ClN4O4S: C 52.72, H 3.96, N 12.94; Found: C 52.62, H 3.86, N 12.90.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)benzamide 9a little yellow solid, yield 75%, m.p. 240–242 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.62 (s, 1H, NH), 11.51 (s, 2H, NH), 10.09 (s, 1H, NH), 7.99~7.96 (m, 2H, ArH), 7.69~7.52 (m, 5H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.01–2.96 (m, 2H, CH2); IR (KBr) ν: 3185, 3081, 1772, 1742, 1655, 1597 cm−1. Anal calcd. for C18H16N4O2S2: C 56.23, H 4.19, N 14.57; Found: C 56.16, H 4.21, N 14.55.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-flurobenzamide 9b little yellow solid, yield 56%, m.p. 238–240 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.57 (s, 1H, NH), 11.59 (s, 1H, NH), 11.51 (s, 1H, NH), 10.10 (s, 1H, NH), 8.09~8.04 (m, 2H, ArH), 7.67~7.64 (m, 2H, ArH), 7.41~7.35 (m, 2H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.01–2.95 (m, 2H, CH2); IR (KBr) ν: 3309, 3047, 1775, 1754, 1658, 1593 cm−1. Anal calcd. for C18H15FN4O2S2: C 53.72, H 3.76, N 13.92; Found: C 53.53, H 3.88, N 13.91.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-chlorobenzamide 9c little yellow solid, yield 58%, m.p. 240–242 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.52 (s, 1H, NH), 11.64 (s, 1H, NH), 11.50 (s, 1H, NH), 10.10 (s, 1H, NH), 8.00~7.97 (m, 2H, ArH), 7.67~7.60 (m, 4H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.01–2.96 (m, 2H, CH2); IR (KBr) ν: 3158, 3077, 1776, 1732, 1617, 1597 cm−1. Anal calcd. for C18H15ClN4O2S2: C 51.61, H 3.61, N 13.37; Found: C 51.46, H 3.72, N 13.47.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-methylbenzamide 9d little yellow solid, yield 70%, m.p. 237–238 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.68 (s, 1H, NH), 11.51 (s, 1H, NH), 11.43 (s, 1H, NH), 10.10 (s, 1H, NH), 7.92~7.89 (m, 2H, ArH), 7.67~7.64 (m, 2H, ArH), 7.36~7.33 (m, 2H, ArH), 7.24~7.21 (m, 2H, ArH), 4.59–4.56 (m, 1H, CH), 3.01–2.95 (m, 2H, CH2), 2.40 (s, 3H, CH3); IR (KBr) ν: 3284, 3047, 1773, 1752, 1655, 1596 cm−1. Anal calcd. for C19H18N4O2S2: C 57.27, H 4.55, N 14.06; Found: C 57.20, H 4.65, N 14.11.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-methoxybenzamide 9e little yellow solid, yield 46%, m.p. 232–234 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.74 (s, 1H, NH), 11.50 (s, 1H, NH), 11.35 (s, 1H, NH), 10.10 (s, 1H, NH), 8.03~8.00 (m, 2H, ArH), 7.67~7.64 (m, 2H, ArH), 7.23~7.20 (m, 2H, ArH), 7.08~7.05 (m, 2H, ArH), 4.59–4.56 (m, 1H, CH), 3.86 (s, 3H, OCH3), 3.01–2.96 (m, 2H, CH2); IR (KBr) ν: 3306, 3051, 1774, 1752, 1668, 1596 cm−1. Anal calcd. for C19H18N4O3S2: C 55.05, H 4.38, N 13.52; Found: C 54.82, H 4.45, N 13.49.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-4-nitrobenzamide 9f little yellow solid, yield 85%, m.p. 244–245 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.40 (s, 1H, NH), 11.91 (s, 1H, NH), 11.50 (s, 1H, NH), 10.10 (s, 1H, NH), 8.36~8.33 (m, 2H, ArH), 8.18~8.15 (m, 2H, ArH), 7.68~7.64 (m, 2H, ArH), 7.25~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.05–2.97 (m, 2H, CH2); IR (KBr) ν: 3202, 3060, 1775, 1746, 1672, 1594 cm−1. Anal calcd. for C18H15N5O4S2: C 50.34, H 3.52, N 16.31; Found: C 50.18, H 3.67, N 16.24.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-methylbenzamide 9g little yellow solid, yield 63%, m.p. 220–222 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.58 (s, 1H, NH), 11.68 (s, 1H, NH), 11.50 (s, 1H, NH), 10.10 (s, 1H, NH), 7.70~7.66 (m, 2H, ArH), 7.51~7.41 (m, 2H, ArH), 7.32~7.27 (m, 2H, ArH), 7.24~7.20 (m, 2H, ArH), 4.59–4.56 (m, 1H, CH), 3.02–2.95 (m, 2H, CH2), 2.42 (s, 3H, CH3); IR (KBr) ν: 3187, 3094, 1774, 1745, 1676, 1587 cm−1. Anal calcd. for C19H18N4O2S2: C 57.27, H 4.55, N 14.06; Found: C 57.14, H 4.62, N 14.35.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-chlorobenzamide 9h little yellow solid, yield 53%, m.p. 216–218 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.38 (s, 1H, NH), 11.98 (s, 1H, NH), 11.51 (s, 1H, NH), 10.10 (s, 1H, NH), 7.68~7.43 (m, 6H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.02–2.96 (m, 2H, CH2); IR (KBr) ν: 3224, 3062, 1776, 1745, 1685, 1593 cm−1. Anal calcd. for C18H15ClN4O2S2: C 51.61, H 3.61, N 13.37; Found: C 51.44, H 3.70, N 13.36.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-methoxybenzamide 9i little yellow solid, yield 69%, m.p. 244–246 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.58 (s, 1H, NH), 11.50 (s, 1H, NH), 11.20 (s, 1H, NH), 10.09 (s, 1H, NH), 7.94~7.91 (m, 1H, ArH), 7.70~7.64 (m, 3H, ArH), 7.31~7.15 (m, 4H, ArH), 4.59–4.56 (m, 1H, CH), 4.01 (s, 3H, OCH3), 3.02–2.94 (m, 2H, CH2); IR (KBr) ν: 3159, 3084, 1775, 1752, 1651, 1595 cm−1. Anal calcd. for C19H18N4O3S2: C 55.05, H 4.38, N 13.52; Found: C 54.92, H 4.47, N 13.63.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-2-nitrobenzamide 9j little yellow solid, yield 54%, m.p. 202–204 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.29 (s, 1H, NH), 12.12 (s, 1H, NH), 11.51 (s, 1H, NH), 10.11 (s, 1H, NH), 8.24~8.21 (m, 1H, ArH), 7.94~7.88 (m, 1H, ArH), 7.81~7.66 (m, 4H, ArH), 7.25~7.22 (m, 2H, ArH), 4.61–4.56 (m, 1H, CH), 3.02–2.96 (m, 2H, CH2); IR (KBr) ν: 3166, 3030, 1775, 1740, 1693, 1598 cm−1. Anal calcd. for C18H15N5O4S2·1/3H2O: C 49.64, H 3.63, N 16.08; Found: C 49.67, H 3.68, N 16.19.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-3-methylbenzamide 9k little yellow solid, yield 88%, m.p. 234–236 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.65 (s, 1H, NH), 11.50 (s, 1H, NH), 11.47 (s, 1H, NH), 10.10 (s, 1H, NH), 7.83~7.65 (m, 4H, ArH), 7.49~7.40 (m, 2H, ArH), 7.24~7.21 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.01–2.94 (m, 2H, CH2), 2.39 (s, 3H, CH3); IR (KBr) ν: 3176, 3051, 1776, 1751, 1657, 1597 cm−1. Anal calcd. for C19H18N4O2S2: C 56.42, H 4.65, N 13.85; Found: C 56.50, H 4.63, N 14.11.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)-3-nitrobenzamide 9l little yellow solid, yield 73%, m.p. 242–243 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.43 (s, 1H, NH), 11.97 (s, 1H, NH), 11.51 (s, 1H, NH), 10.10 (s, 1H, NH), 8.78~8.76 (m, 1H, ArH), 8.50~8.47 (m, 1H, ArH), 8.38~8.35 (m, 1H, ArH), 7.86~7.81 (m, 1H, ArH), 7.68~7.65 (m, 2H, ArH), 7.25~7.22 (m, 2H, ArH), 4.60–4.56 (m, 1H, CH), 3.02–2.96 (m, 2H, CH2); IR (KBr) ν: 3172, 3090, 1774, 1738, 1692, 1604 cm−1. Anal calcd. for C18H15N5O4S2: C 50.34, H 3.52, N 16.31; Found: C 49.94, H 3.68, N 16.26.
N-((4-((4-oxo-2-thioxoimidazolidin-5-yl)methyl)phenyl)carbamothioyl)thiophene-2-carboxamide 9m little yellow solid, yield 68%, m.p. 242–244 ºC, 1H NMR (DMSO-d6, 300 MHz) δ: 12.48 (s, 1H, NH), 11.58 (s, 1H, NH), 11.51 (s, 1H, NH), 10.10 (s, 1H, NH), 8.39–8.37 (m, 1H, ThH), 8.06–8.04 (m, 1H, ThH), 7.66~7.63 (m, 2H, ArH), 7.27~7.20 (m, 3H, ArH + ThH), 4.60–4.55 (m, 1H, CH), 3.01–2.95 (m, 2H, CH2); IR (KBr) ν: 3176, 3107, 1776, 1737, 1668, 1599 cm−1. Anal calcd. for C16H14N4O2S3: C 49.21, H 3.61, N 14.35; Found: C 49.16, H 3.72, N 14.52.