Inhibiting Invasion into Human Bladder Carcinoma 5637 Cells with Diallyl Trisulfide by Inhibiting Matrix Metalloproteinase Activities and Tightening Tight Junctions
Abstract
:1. Introduction
2. Results and Discussion
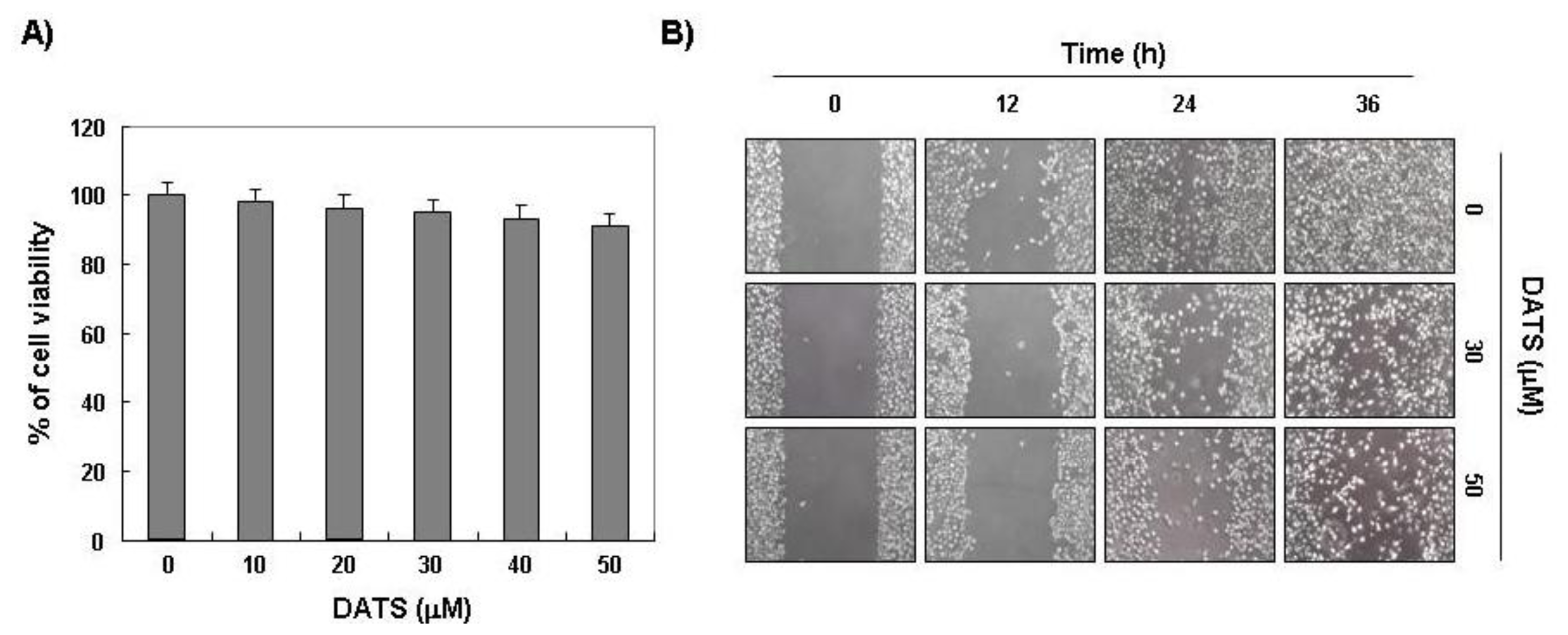
2.1. DATS Inhibits 5637 Cell Migration and Invasion
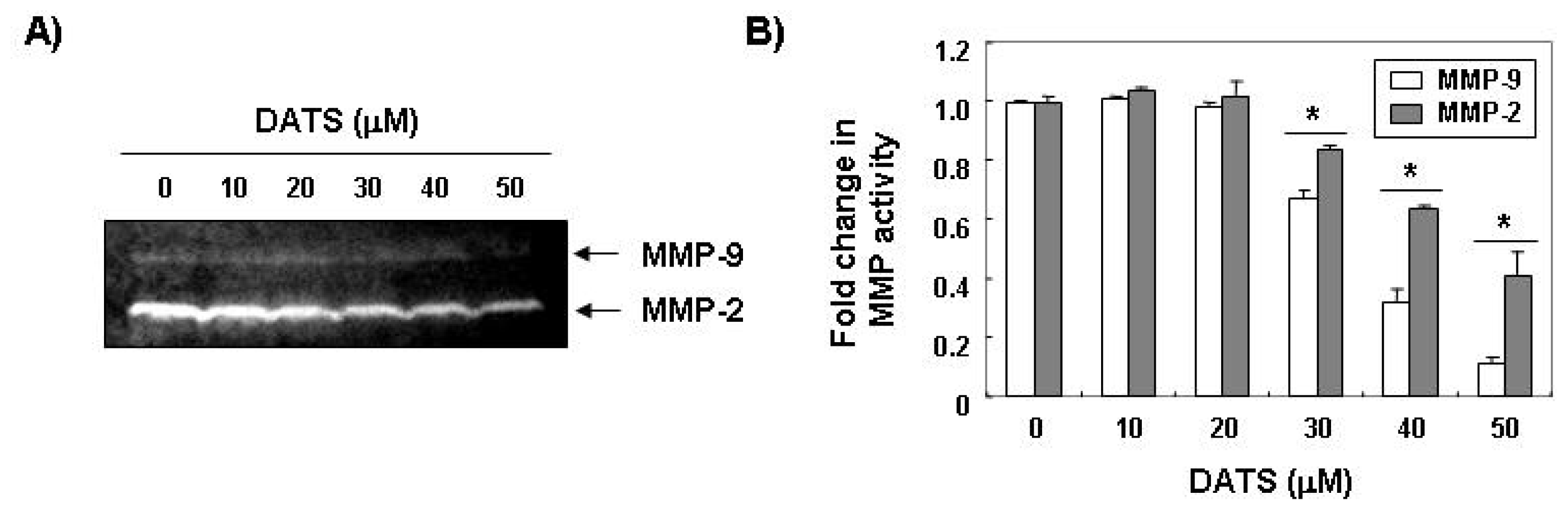
2.2. DATS Inhibits MMP Expression and Activity
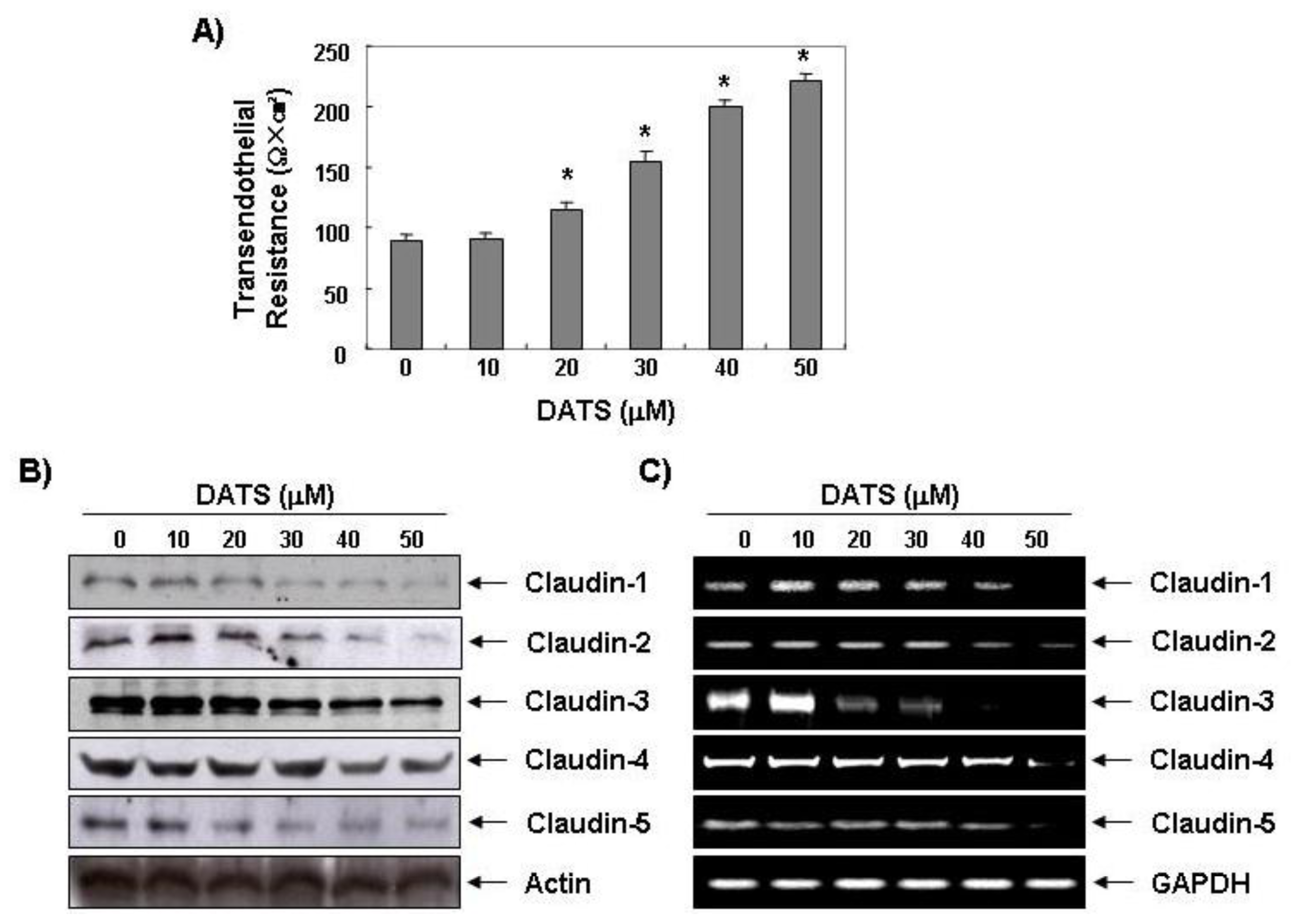
2.3. DATS Enhances the Transepithelial Electrical Resistance (TER)
2.4. DATS Inhibits Claudin Family Member Expression
3. Experimental Section
3.1. Cell Culture and Growth Inhibition Assay
3.2. Wound Healing Assay
3.3. Cell Invasion Assay
3.4. Western Blot Analysis
3.5. RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (PCR)
3.6. Gelatin Zymography
3.7. In Vitro MMP Activity Assay
3.8. TER Measurements
3.9. Statistics
4. Conclusions

Acknowledgments
Conflicts of Interest
References
- Parkin, D.M. The global burden of urinary bladder cancer. Scand. J. Urol. Nephrol. Suppl 2008, 218, 12–20. [Google Scholar]
- Taylor, J.A., III; Kuchel, G.A. Bladder cancer in the elderly: Clinical outcomes, basic mechanisms, and future research direction. Nat. Clin. Pract. Urol 2009, 6, 135–144. [Google Scholar]
- Smaldone, M.C.; Jacobs, B.L.; Smaldone, A.M.; Hrebinko, R.L., Jr. Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology 2008, 72, 613–616. [Google Scholar]
- Galsky, M.D.; Hall, S.J. Bladder cancer: Current management and opportunities for a personalized approach. Mt. Sinai J. Med 2010, 77, 587–596. [Google Scholar]
- Nargund, V.H.; Tanabalan, C.K.; Kabir, M.N. Management of non-muscle-invasive (superficial) bladder cancer. Semin. Oncol 2012, 39, 559–572. [Google Scholar]
- Kamat, A.M.; Lamm, D.L. Chemoprevention of bladder cancer. Urol. Clin. North Am 2002, 29, 157–168. [Google Scholar]
- Moyad, M.A. Bladder cancer recurrence: Part II. What do I tell my patients about lifestyle changes and dietary supplements? Curr. Opin. Urol 2003, 13, 379–383. [Google Scholar]
- Lai, K.C.; Kuo, C.L.; Ho, H.C.; Yang, J.S.; Ma, C.Y.; Lu, H.F.; Huang, H.Y.; Chueh, F.S.; Yu, C.C.; Chung, J.G. Diallyl sulfide, diallyl disulfide and diallyl trisulfide affect drug resistant gene expression in colo 205 human colon cancer cells in vitro and in vivo. Phytomedicine 2012, 19, 625–630. [Google Scholar]
- Na, H.K.; Kim, E.H.; Choi, M.A.; Park, J.M.; Kim, D.H.; Surh, Y.J. Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem. Pharmacol 2012, 84, 1241–1250. [Google Scholar]
- Xiao, D.; Lew, K.L.; Kim, Y.A.; Zeng, Y.; Hahm, E.R.; Dhir, R.; Singh, S.V. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin. Cancer Res 2006, 12, 6836–6843. [Google Scholar]
- Choi, Y.H.; Park, H.S. Apoptosis induction of U937 human leukemia cells by diallyl trisulfide induces through generation of reactive oxygen species. J. Biomed. Sci 2012, 19, 50. [Google Scholar]
- Xiao, D.; Herman-Antosiewicz, A.; Antosiewicz, J.; Xiao, H.; Brisson, M.; Lazo, J.S.; Singh, S.V. Diallyl trisulfide-induced G(2)-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc25C. Oncogene 2005, 24, 6256–6268. [Google Scholar]
- Xiao, D.; Zeng, Y.; Singh, S.V. Diallyl trisulfide-induced apoptosis in human cancer cells is linked to checkpoint kinase 1-mediated mitotic arrest. Mol. Carcinog 2009, 48, 1018–1029. [Google Scholar]
- Li, W.; Tian, H.; Li, L.; Li, S.; Yue, W.; Chen, Z.; Qi, L.; Hu, W.; Zhu, Y.; Hao, B.; et al. Diallyl trisulfide induces apoptosis and inhibits proliferation of A549 cells in vitro and in vivo. Acta Biochim. Biophys. Sin. 2012, 44, 577–583. [Google Scholar]
- Zhang, Z.M.; Yang, X.Y.; Deng, S.H.; Xu, W.; Gao, H.Q. Anti-tumor effects of polybutylcyanoacrylate nanoparticles of diallyl trisulfide on orthotopic transplantation tumor model of hepatocellular carcinoma in BALB/c nude mice. Chin. Med. J. (Engl.) 2007, 120, 1336–1342. [Google Scholar]
- Wu, P.P.; Liu, K.C.; Huang, W.W.; Chueh, F.S.; Ko, Y.C.; Chiu, T.H.; Lin, J.P.; Kuo, J.H.; Yang, J.S.; Chung, J.G. Diallyl trisulfide (DATS) inhibits mouse colon tumor in mouse CT-26 cells allograft model in vivo. Phytomedicine 2011, 18, 672–676. [Google Scholar]
- Lai, K.C.; Hsu, S.C.; Kuo, C.L.; Yang, J.S.; Ma, C.Y.; Lu, H.F.; Tang, N.Y.; Hsia, T.C.; Ho, H.C.; Chung, J.G. Diallyl sulfide, diallyl disulfide, and diallyl trisulfide inhibit migration and invasion in human colon cancer colo 205 cells through the inhibition of matrix metalloproteinase-2, -7, and -9 expressions. Environ. Toxicol 2011. [Google Scholar] [CrossRef]
- Engbring, J.A.; Kleinman, H.K. The basement membrane matrix in malignancy. J. Pathol 2003, 200, 465–470. [Google Scholar]
- Brooks, S.A.; Lomax-Browne, H.J.; Carter, T.M.; Kinch, C.E.; Hall, D.M. Molecular interactions in cancer cell metastasis. Acta Histochem 2010, 112, 3–25. [Google Scholar]
- Gibbs, D.F.; Warner, R.L.; Weiss, S.J.; Johnson, K.J.; Varani, J. Characterization of matrix metalloproteinases produced by rat alveolar macrophages. Am. J. Respir. Cell Mol. Biol 1999, 20, 1136–1144. [Google Scholar]
- Mook, O.R.; Frederiks, W.M.; Van Noorden, C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 2004, 1705, 69–89. [Google Scholar]
- Lambert, E.; Dasse, E.; Haye, B.; Petitfrere, E. TIMPs as multifacial proteins. Crit. Rev. Oncol. Hematol 2004, 49, 187–198. [Google Scholar]
- Kim, Y.S.; Kim, S.H.; Kang, J.G.; Ko, J.H. Expression level and glycan dynamics determine the net effects of TIMP-1 on cancer progression. BMB Rep 2012, 45, 623–628. [Google Scholar]
- Soler, A.P.; Miller, R.D.; Laughlin, K.V.; Carp, N.Z.; Klurfeld, D.M.; Mullin, J.M. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 1999, 20, 1425–1431. [Google Scholar]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol 2004, 286, C1213–C1228. [Google Scholar]
- Turksen, K.; Troy, T.C. Junctions gone bad: Claudins and loss of the barrier in cancer. Biochim. Biophys. Acta 2011, 1816, 73–79. [Google Scholar]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta 2008, 1778, 631–645. [Google Scholar]
- Singh, A.B.; Sharma, A.; Dhawan, P. Claudin family of proteins and cancer: An overview. J. Oncol 2010, 2010, 541957. [Google Scholar]
- Stebbing, J.; Filipović, A.; Giamas, G. Claudin-1 as a promoter of EMT in hepatocellular carcinoma. Oncogene 2013. [Google Scholar] [CrossRef]
- Rangel, L.B.; Agarwal, R.; D’Souza, T.; Pizer, E.S.; Alò, P.L.; Lancaster, W.D.; Gregoire, L.; Schwartz, D.R.; Cho, K.R.; Morin, P.J. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin. Cancer Res 2003, 9, 2567–2575. [Google Scholar]
- Shang, X.; Lin, X.; Alvarez, E.; Manorek, G.; Howell, S.B. Tight junction proteins claudin-3 and claudin-4 control tumor growth and metastases. Neoplasia 2012, 14, 974–985. [Google Scholar]
- Agarwal, R.; D’Souza, T.; Morin, P.J. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res 2005, 65, 7378–7385. [Google Scholar]
- Yoon, C.H.; Kim, M.J.; Park, M.J.; Park, I.C.; Hwang, S.G.; An, S.; Choi, Y.H.; Yoon, G.; Lee, S.J. Claudin-1 acts through c-Abl-protein kinase Cdelta (PKCδ) signaling and has a causal role in the acquisition of invasive capacity in human liver cells. J. Biol. Chem 2010, 285, 226–233. [Google Scholar]
- Lin, X.; Shang, X.; Manorek, G.; Howell, S.B. Regulation of the epithelial-mesenchymal transition by claudin-3 and claudin-4. PLoS One 2013, 8, e67496. [Google Scholar]
- Liu, Y.; Miao, Y.; Wang, J.; Lin, X.; Wang, L.; Xu, H.T.; Wang, E.H. DEC1 is positively associated with the malignant phenotype of invasive breast cancers and negatively correlated with the expression of claudin-1. Int. J. Mol. Med 2013, 31, 855–860. [Google Scholar]
- Park, H.S.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Hwang, H.J.; Choi, Y.W.; Choi, Y.H. Inhibition of matrix metalloproteinase activities and tightening of tight junctions by diallyl disulfide in AGS human gastric carcinoma cells. J. Food Sci 2011, 76, T105–T111. [Google Scholar]
- Chen, C.; Pung, D.; Leong, V.; Hebbar, V.; Shen, G.; Nair, S.; Li, W.; Kong, A.N. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: Effect of chemical structure and stress signals. Free Radic. Biol. Med 2004, 37, 1578–1590. [Google Scholar]
- Liu, K.L.; Chen, H.W.; Wang, R.Y.; Lei, Y.P.; Sheen, L.Y.; Lii, C.K. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-kappaB activation in RAW 264.7 macrophages. J. Agric. Food. Chem 2006, 54, 3472–3478. [Google Scholar]
- Zhao, H.; Yang, Z.; Wang, X.; Zhang, X.; Wang, M.; Wang, Y.; Mei, Q.; Wang, Z. Triptolide inhibits ovarian cancer cell invasion by repression of matrix metalloproteinase 7 and 19 and upregulation of E-cadherin. Exp. Mol. Med 2012, 44, 633–641. [Google Scholar]
- Koh, P.O. Gingko biloba extract (EGb 761) attenuates ischemic brain injury-induced reduction in Ca2+ sensor protein hippocalcin. Lab. Anim. Res 2012, 28, 199–204. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shin, D.Y.; Cha, H.-J.; Kim, G.-Y.; Kim, W.-J.; Choi, Y.H. Inhibiting Invasion into Human Bladder Carcinoma 5637 Cells with Diallyl Trisulfide by Inhibiting Matrix Metalloproteinase Activities and Tightening Tight Junctions. Int. J. Mol. Sci. 2013, 14, 19911-19922. https://doi.org/10.3390/ijms141019911
Shin DY, Cha H-J, Kim G-Y, Kim W-J, Choi YH. Inhibiting Invasion into Human Bladder Carcinoma 5637 Cells with Diallyl Trisulfide by Inhibiting Matrix Metalloproteinase Activities and Tightening Tight Junctions. International Journal of Molecular Sciences. 2013; 14(10):19911-19922. https://doi.org/10.3390/ijms141019911
Chicago/Turabian StyleShin, Dong Yeok, Hee-Jae Cha, Gi-Young Kim, Wun-Jae Kim, and Yung Hyun Choi. 2013. "Inhibiting Invasion into Human Bladder Carcinoma 5637 Cells with Diallyl Trisulfide by Inhibiting Matrix Metalloproteinase Activities and Tightening Tight Junctions" International Journal of Molecular Sciences 14, no. 10: 19911-19922. https://doi.org/10.3390/ijms141019911




