Targeting of Rho Kinase Ameliorates Impairment of Diabetic Endothelial Function in Intrarenal Artery
Abstract
:1. Introduction
2. Results and Discussion
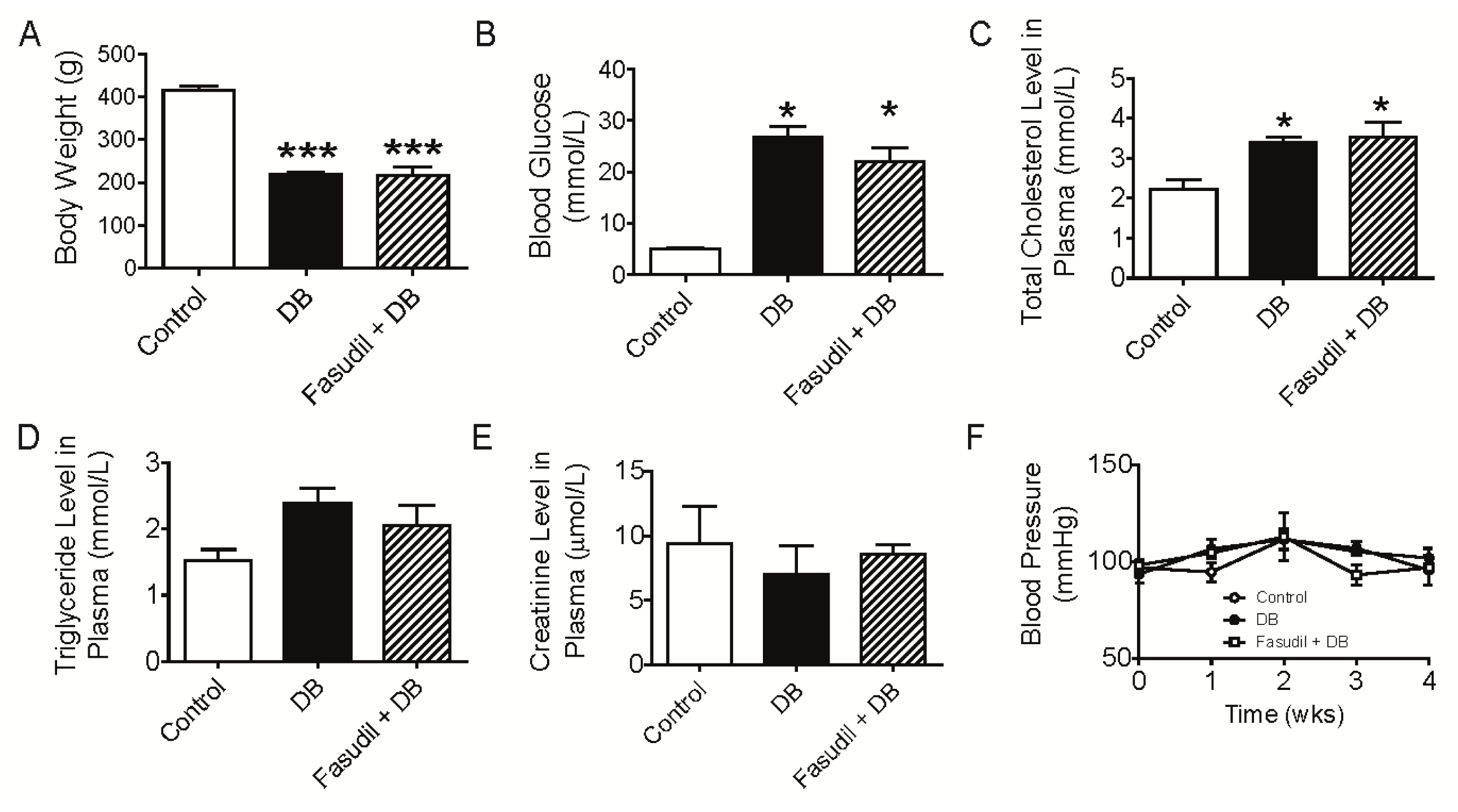
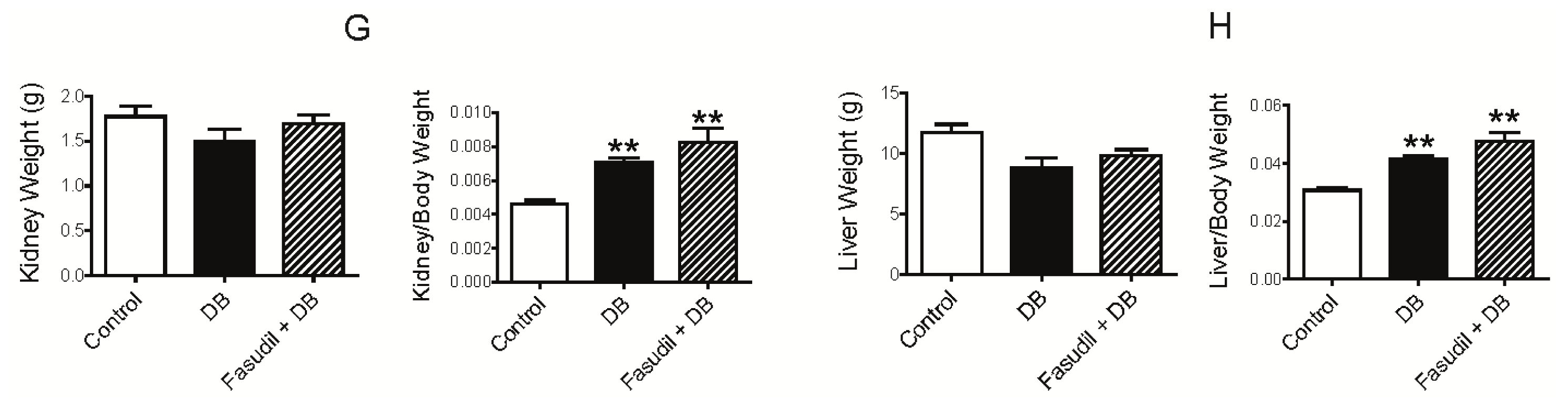
2.1. Chronic Rho Kinase Inhibition Does Not Meliorate the Metabolic Parameters in Diabetic Rats
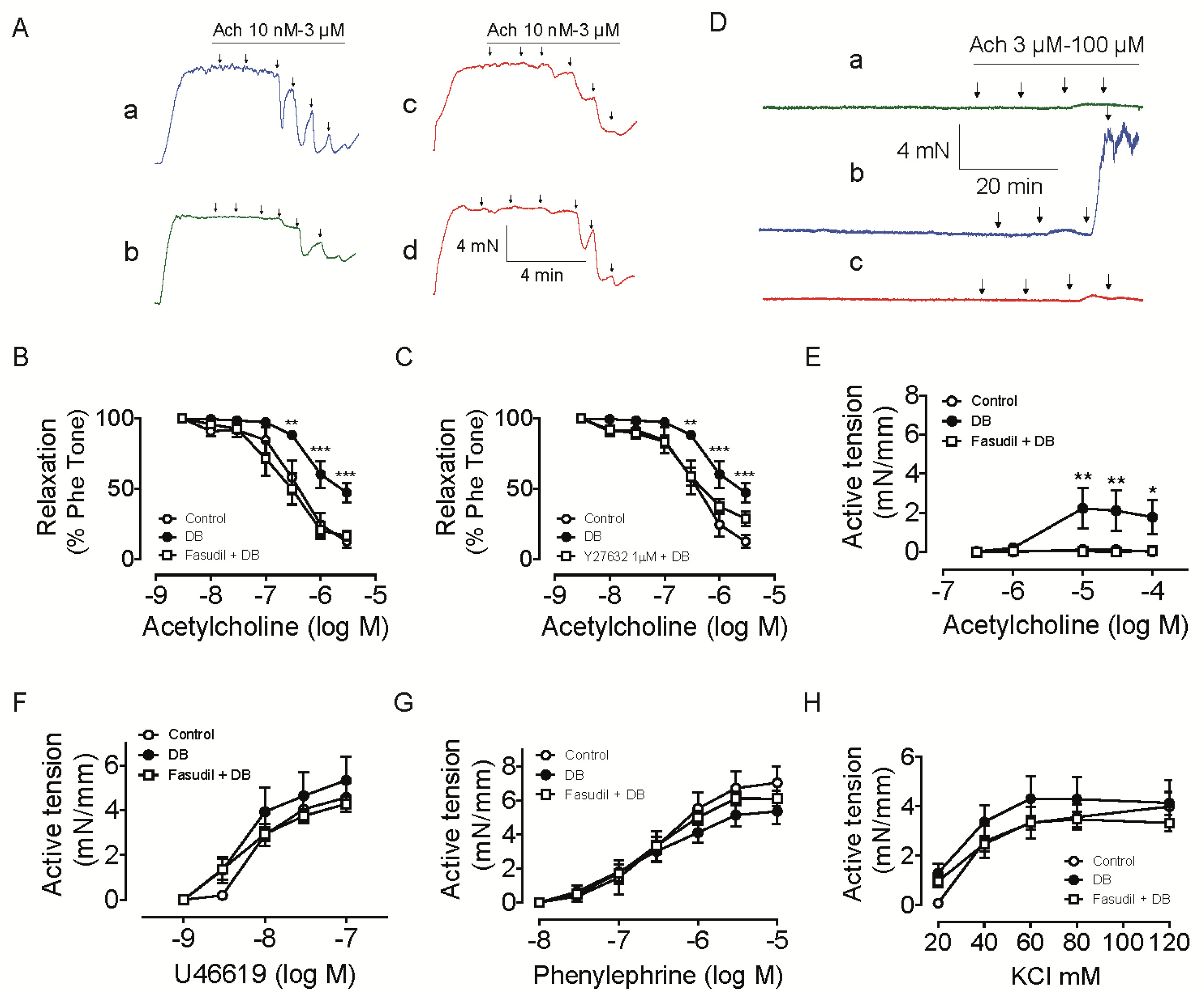
2.2. Chronic Rho Kinase Inhibition Improves Endothelial Function in Intrarenal Artery from Diabetic Rats
2.2.1. Effect of Rho Kinase Inhibition on Endothelium-Dependent Relaxation in Intrarenal Artery
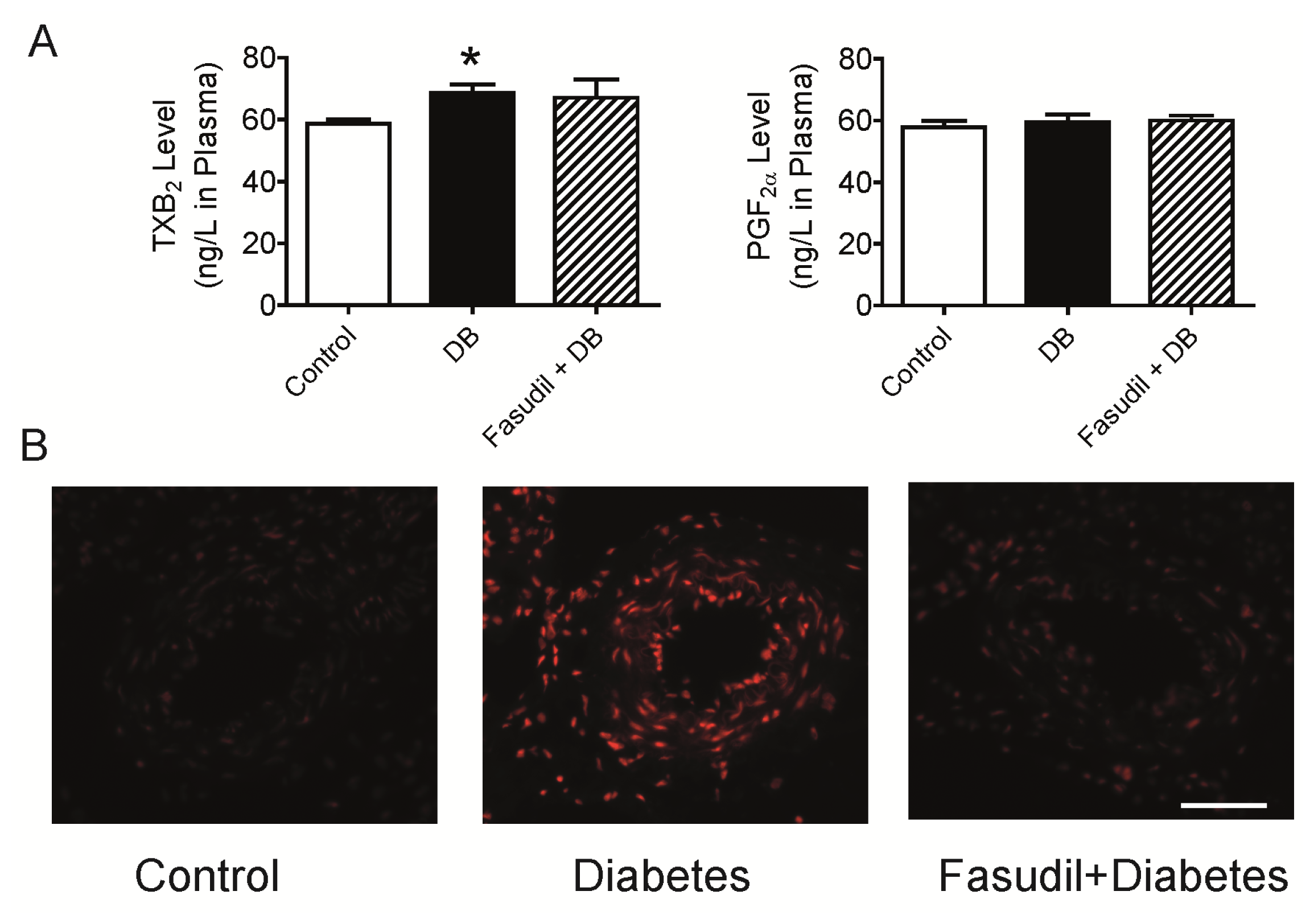
2.2.2. Effect of Rho Kinase Inhibition on Endothelium-Dependent Contraction in Intrarenal Artery
2.2.3. Effect of Rho Kinase Inhibition on O2•− Production in the Intrarenal Artery
2.3. Chronic Rho Kinase Inhibition Ameliorates Development of Diabetic Nephropathy in Renal Cortex from Diabetic Rats
2.3.1. Effect of Rho Kinase Inhibition on Mesangial Expansion and Glomerular hypertrophy
2.3.2. Effect of Rho Kinase Inhibition on NOX4 Expression in Renal Cortex
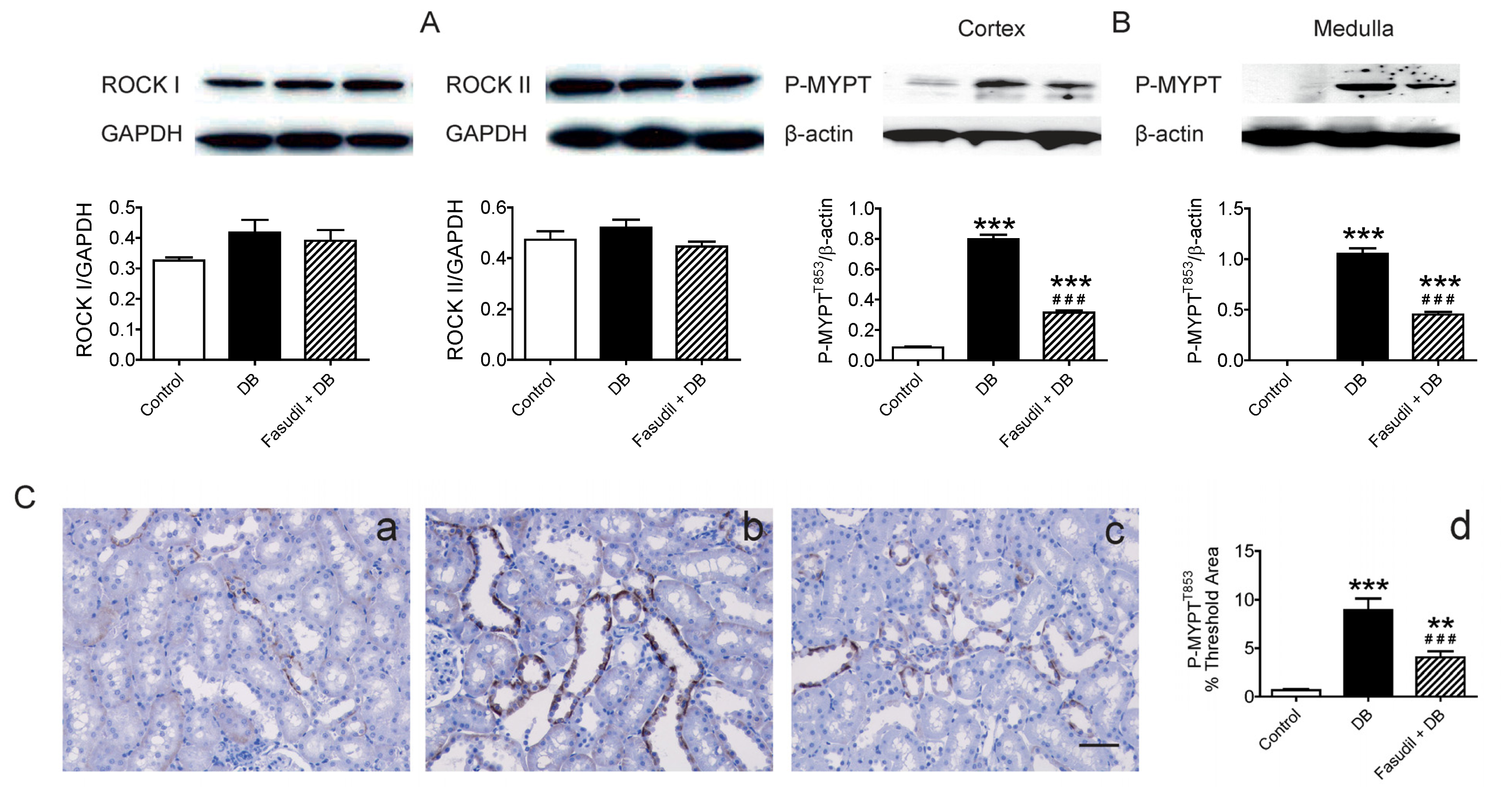
2.4. Chronic Rho Kinase Inhibition Alters No Rho-Kinase Expression but Decreased Rho Kinase Activity in the Kidney
3. Experimental Section
3.1. Animal Model
3.2. Measurement of Blood Pressure and Plasma Analysis
3.3. Vascular Reactivity
3.4. Immunoblotting
3.5. Histology and Immunohistochemistry
3.6. Quantitative RT-PCR
3.7. Localization and Quantification of Superoxide Anion by Dihydroethidium
3.8. Chemicals
3.9. Data Analysis
4. Conclusions


| Primer | Forward oligonucleotides | Reverse oligonucleotides |
|---|---|---|
| P22phox | CCTCCACTTACTGCTGTCCG | GTAGGTGGCTGCTTGATGGT |
| P47phox | GTCGGAGAAGGTGGTCTACAG | TCTTCACCTGGCTGTCATTGG |
| Gp91phox | CTGCCAGTGTGTCGGAATCT | TGTGAATGGCCGTGTGAAGT |
| Nox3 | GCTGGGATGAATACCAGGCA | GCTGCTGCTAGGGTGATTGT |
| Nox4 | CTGACAGGTGTCTGCATGGT | ACTTCAACAAGCCACCCGAA |
| β-actin | AGATCAAGATCATTGCTCCTCCT | ACGCAGCTCAGTAACAGTCC |
Acknowledgments
Conflicts of Interest
References
- Yao, L.; Chandra, S.; Toque, H.A.; Bhatta, A.; Rojas, M.; Caldwell, R.B.; Caldwell, R.W. Prevention of diabetes-induced arginase activation and vascular dysfunction by rho kinase (rock) knockout. Cardiovasc. Res 2013, 97, 509–519. [Google Scholar]
- Sakurada, S.; Okamoto, H.; Takuwa, N.; Sugimoto, N.; Takuwa, Y. Rho activation in excitatory agonist-stimulated vascular smooth muscle. Am. J. Physiol. Cell Physiol 2001, 281, C571–C578. [Google Scholar]
- Wilson, D.P.; Susnjar, M.; Kiss, E.; Sutherland, C.; Walsh, M.P. Thromboxane a2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of mypt1 at thr-855, but not thr-697. Biochem. J 2005, 389, 763–774. [Google Scholar]
- Ming, X.F.; Viswambharan, H.; Barandier, C.; Ruffieux, J.; Kaibuchi, K.; Rusconi, S.; Yang, Z. Rho gtpase/rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase b/akt in human endothelial cells. Mol. Cell Biol 2002, 22, 8467–8477. [Google Scholar]
- Shiga, N.; Hirano, K.; Hirano, M.; Nishimura, J.; Nawata, H.; Kanaide, H. Long-term inhibition of rhoa attenuates vascular contractility by enhancing endothelial no production in an intact rabbit mesenteric artery. Circ. Res 2005, 96, 1014–1021. [Google Scholar]
- Guagnini, F.; Ferazzini, M.; Grasso, M.; Blanco, S.; Croci, T. Erectile properties of the rho-kinase inhibitor sar407899 in diabetic animals and human isolated corpora cavernosa. J. Transl. Med 2012, 10, 59. [Google Scholar]
- Li, H.; Peng, W.; Jian, W.; Li, Y.; Li, Q.; Li, W.; Xu, Y. Rock inhibitor fasudil attenuated high glucose-induced mcp-1 and vcam-1 expression and monocyte-endothelial cell adhesion. Cardiovasc. Diabetol 2012, 11, 65. [Google Scholar]
- Mishra, R.K.; Alokam, R.; Sriram, D.; Yogeeswari, P. Potential role of rho kinase inhibitors in combating diabetes-related complications including diabetic neuropathy—A review. Curr. Diabetes Rev 2013, 9, 249–266. [Google Scholar]
- Guan, S.J.; Ma, Z.H.; Wu, Y.L.; Zhang, J.P.; Liang, F.; Weiss, J.W.; Guo, Q.Y.; Wang, J.Y.; Ji, E.S.; Chu, L. Long-term administration of fasudil improves cardiomyopathy in streptozotocin-induced diabetic rats. Food Chem. Toxicol 2012, 50, 1874–1882. [Google Scholar]
- Arita, R.; Hata, Y.; Nakao, S.; Kita, T.; Miura, M.; Kawahara, S.; Zandi, S.; Almulki, L.; Tayyari, F.; Shimokawa, H.; et al. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes 2009, 58, 215–226. [Google Scholar]
- Zhou, H.; Li, Y.J. Rho kinase inhibitors: Potential treatments for diabetes and diabetic complications. Curr. Pharm. Des 2012, 18, 2964–2973. [Google Scholar]
- Eleftheriadis, T.; Antoniadi, G.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. The renal endothelium in diabetic nephropathy. Ren. Fail 2013, 35, 592–599. [Google Scholar]
- Gojo, A.; Utsunomiya, K.; Taniguchi, K.; Yokota, T.; Ishizawa, S.; Kanazawa, Y.; Kurata, H.; Tajima, N. The rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharmacol 2007, 568, 242–247. [Google Scholar]
- Kikuchi, Y.; Yamada, M.; Imakiire, T.; Kushiyama, T.; Higashi, K.; Hyodo, N.; Yamamoto, K.; Oda, T.; Suzuki, S.; Miura, S. A rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J. Endocrinol 2007, 192, 595–603. [Google Scholar]
- Sun, G.P.; Kohno, M.; Guo, P.; Nagai, Y.; Miyata, K.; Fan, Y.Y.; Kimura, S.; Kiyomoto, H.; Ohmori, K.; Li, D.T.; et al. Involvements of rho-kinase and tgf-beta pathways in aldosterone-induced renal injury. J. Am. Soc. Nephrol 2006, 17, 2193–2201. [Google Scholar]
- Sharpe, C.C.; Hendry, B.M. Signaling: Focus on rho in renal disease. J. Am. Soc. Nephrol 2003, 14, 261–264. [Google Scholar]
- Cicek, F.A.; Kandilci, H.B.; Turan, B. Role of rock upregulation in endothelial and smooth muscle vascular functions in diabetic rat aorta. Cardiovasc. Diabetol 2013, 12, 51. [Google Scholar]
- Komers, R.; Oyama, T.T.; Beard, D.R.; Anderson, S. Effects of systemic inhibition of rho kinase on blood pressure and renal haemodynamics in diabetic rats. Br. J. Pharmacol 2011, 162, 163–174. [Google Scholar]
- Komers, R.; Oyama, T.T.; Beard, D.R.; Tikellis, C.; Xu, B.; Lotspeich, D.F.; Anderson, S. Rho kinase inhibition protects kidneys from diabetic nephropathy without reducing blood pressure. Kidney Int 2011, 79, 432–442. [Google Scholar]
- Salum, E.; Kampus, P.; Zilmer, M.; Eha, J.; Butlin, M.; Avolio, A.P.; Podramagi, T.; Arend, A.; Aunapuu, M.; Kals, J. Effect of vitamin d on aortic remodeling in streptozotocin-induced diabetes. Cardiovasc. Diabetol 2012, 11, 58. [Google Scholar]
- Liu, C.Q.; Leung, F.P.; Wong, S.L.; Wong, W.T.; Lau, C.W.; Lu, L.; Yao, X.; Yao, T.; Huang, Y. Thromboxane prostanoid receptor activation impairs endothelial nitric oxide-dependent vasorelaxations: The role of rho kinase. Biochem. Pharmacol 2009, 78, 374–381. [Google Scholar]
- Liu, C.Q.; Wong, S.L.; Leung, F.P.; Tian, X.Y.; Lau, C.W.; Lu, L.; Yao, X.; Chen, Z.Y.; Yao, T.; Huang, Y. Prostanoid tp receptor-mediated impairment of cyclic amp-dependent vasorelaxation is reversed by phosphodiesterase inhibitors. Eur. J. Pharmacol 2010, 632, 45–51. [Google Scholar]
- Vanhoutte, P.M. Endothelium-dependent contractions in hypertension: When prostacyclin becomes ugly. Hypertension 2011, 57, 526–531. [Google Scholar]
- Feletou, M.; Huang, Y.; Vanhoutte, P.M. Endothelium-mediated control of vascular tone: Cox-1 and cox-2 products. Br. J. Pharmacol 2011, 164, 894–912. [Google Scholar]
- Wong, S.L.; Leung, F.P.; Lau, C.W.; Au, C.L.; Yung, L.M.; Yao, X.; Chen, Z.Y.; Vanhoutte, P.M.; Gollasch, M.; Huang, Y. Cyclooxygenase-2-derived prostaglandin f2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ. Res 2009, 104, 228–235. [Google Scholar]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 2009, 32, S314–S321. [Google Scholar]
- El-Remessy, A.B.; Tawfik, H.E.; Matragoon, S.; Pillai, B.; Caldwell, R.B.; Caldwell, R.W. Peroxynitrite mediates diabetes-induced endothelial dysfunction: Possible role of rho kinase activation. Exp. Diabetes Res 2010, 2010, 247861. [Google Scholar]
- Kizub, I.V.; Pavlova, O.O.; Johnson, C.D.; Soloviev, A.I.; Zholos, A.V. Rho kinase and protein kinase c involvement in vascular smooth muscle myofilament calcium sensitization in arteries from diabetic rats. Br. J. Pharmacol 2010, 159, 1724–1731. [Google Scholar]
- Shah, D.I.; Singh, M. Involvement of rho-kinase in experimental vascular endothelial dysfunction. Mol. Cell. Biochem 2006, 283, 191–199. [Google Scholar]
- Mita, S.; Kobayashi, N.; Yoshida, K.; Nakano, S.; Matsuoka, H. Cardioprotective mechanisms of rho-kinase inhibition associated with enos and oxidative stress-lox-1 pathway in dahl salt-sensitive hypertensive rats. J. Hypertens 2005, 23, 87–96. [Google Scholar]
- Matoba, K.; Kawanami, D.; Okada, R.; Tsukamoto, M.; Kinoshita, J.; Ito, T.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Murai, N.; et al. Rho-kinase inhibition prevents the progression of diabetic nephropathy by downregulating hypoxia-inducible factor 1alpha. Kidney Int 2013, 84, 545–554. [Google Scholar]
- Lee, D.Y.; Wauquier, F.; Eid, A.A.; Roman, L.J.; Ghosh Choudhury, G.; Khazim, K.; Block, K.; Gorin, Y. Nox4 nadph oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric oxide synthase and fibronectin expression in response to angiotensin ii. Role of mitochondrial reactive oxygen species. J. Biol. Chem 2013. [Google Scholar] [CrossRef]
- Nunes, K.P.; Rigsby, C.S.; Webb, R.C. Rhoa/rho-kinase and vascular diseases: What is the link? Cell. Mol. Life Sci 2010, 67, 3823–3836. [Google Scholar]
- Xie, Z.; Su, W.; Guo, Z.; Pang, H.; Post, S.R.; Gong, M.C. Up-regulation of cpi-17 phosphorylation in diabetic vasculature and high glucose cultured vascular smooth muscle cells. Cardiovasc. Res 2006, 69, 491–501. [Google Scholar]
- Rasineni, K.; Bellamkonda, R.; Singareddy, S.R.; Desireddy, S. Antihyperglycemic activity of catharanthus roseus leaf powder in streptozotocin-induced diabetic rats. Pharmacognosy Res 2010, 2, 195–201. [Google Scholar]
- Tsounapi, P.; Saito, M.; Kitatani, K.; Dimitriadis, F.; Ohmasa, F.; Shimizu, S.; Kinoshita, Y.; Takenaka, A.; Satoh, K. Fasudil improves the endothelial dysfunction in the aorta of spontaneously hypertensive rats. Eur. J. Pharmacol 2012, 691, 182–189. [Google Scholar]
- Leung, F.P.; Yao, X.; Lau, C.W.; Ko, W.H.; Lu, L.; Huang, Y. Raloxifene relaxes rat intrarenal arteries by inhibiting Ca2+ influx. Am. J. Physiol. Ren. Physiol 2005, 289, F137–F144. [Google Scholar]
- Liu, C.; Desikan, R.; Ying, Z.; Gushchina, L.; Kampfrath, T.; Deiuliis, J.; Wang, A.; Xu, X.; Zhong, J.; Rao, X.; et al. Effects of a novel pharmacologic inhibitor of myeloperoxidase in a mouse atherosclerosis model. PLoS One 2012, 7, e50767. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yin, H.; Ru, H.; Yu, L.; Kang, Y.; Lin, G.; Liu, C.; Sun, L.; Shi, L.; Sun, Q.; Liu, C. Targeting of Rho Kinase Ameliorates Impairment of Diabetic Endothelial Function in Intrarenal Artery. Int. J. Mol. Sci. 2013, 14, 20282-20298. https://doi.org/10.3390/ijms141020282
Yin H, Ru H, Yu L, Kang Y, Lin G, Liu C, Sun L, Shi L, Sun Q, Liu C. Targeting of Rho Kinase Ameliorates Impairment of Diabetic Endothelial Function in Intrarenal Artery. International Journal of Molecular Sciences. 2013; 14(10):20282-20298. https://doi.org/10.3390/ijms141020282
Chicago/Turabian StyleYin, Hongping, Hailong Ru, Liping Yu, Yanhua Kang, Guohua Lin, Chuanfei Liu, Lixian Sun, Liyun Shi, Qinghua Sun, and Cuiqing Liu. 2013. "Targeting of Rho Kinase Ameliorates Impairment of Diabetic Endothelial Function in Intrarenal Artery" International Journal of Molecular Sciences 14, no. 10: 20282-20298. https://doi.org/10.3390/ijms141020282




