Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Enrolled Sarcoidosis Patients
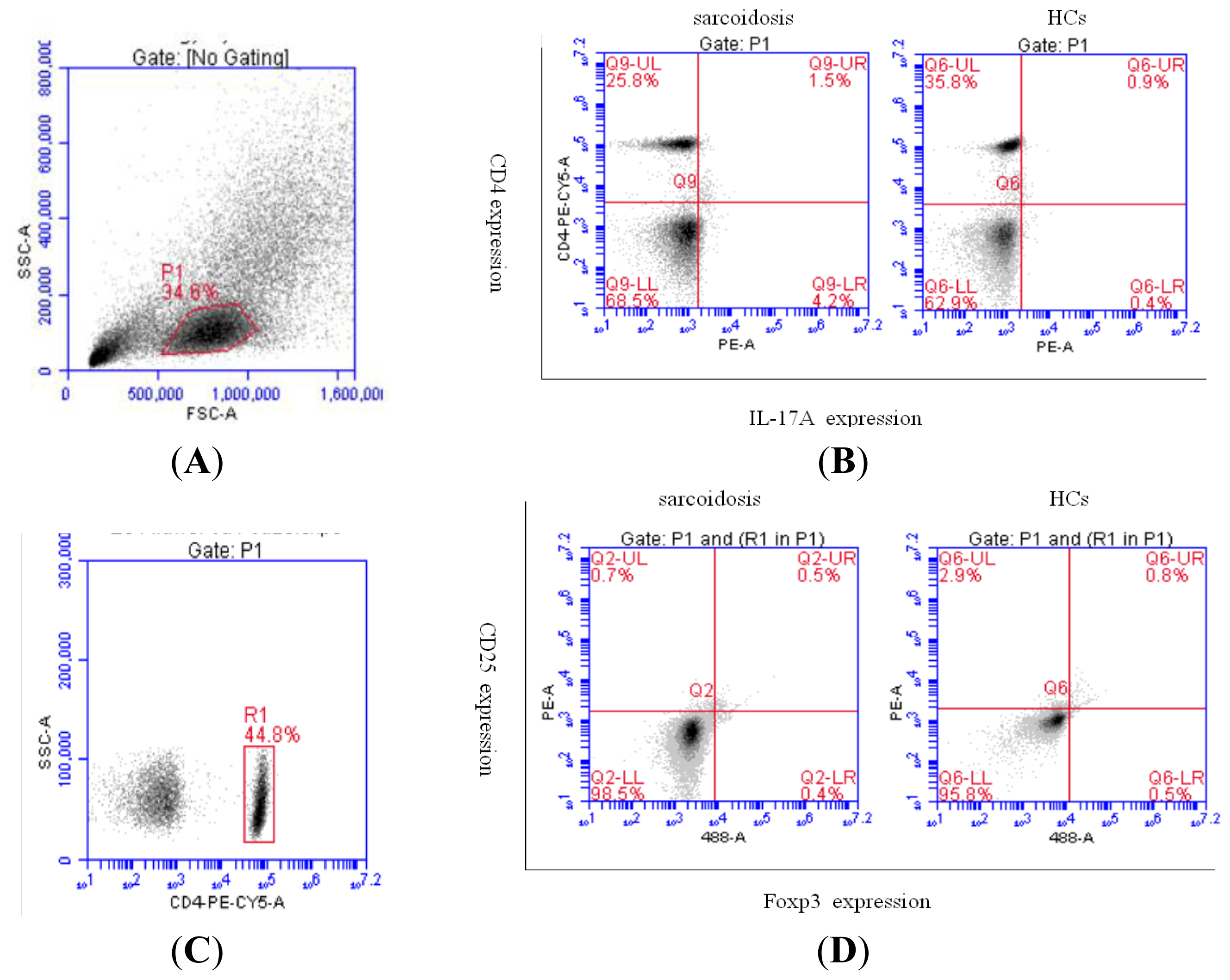
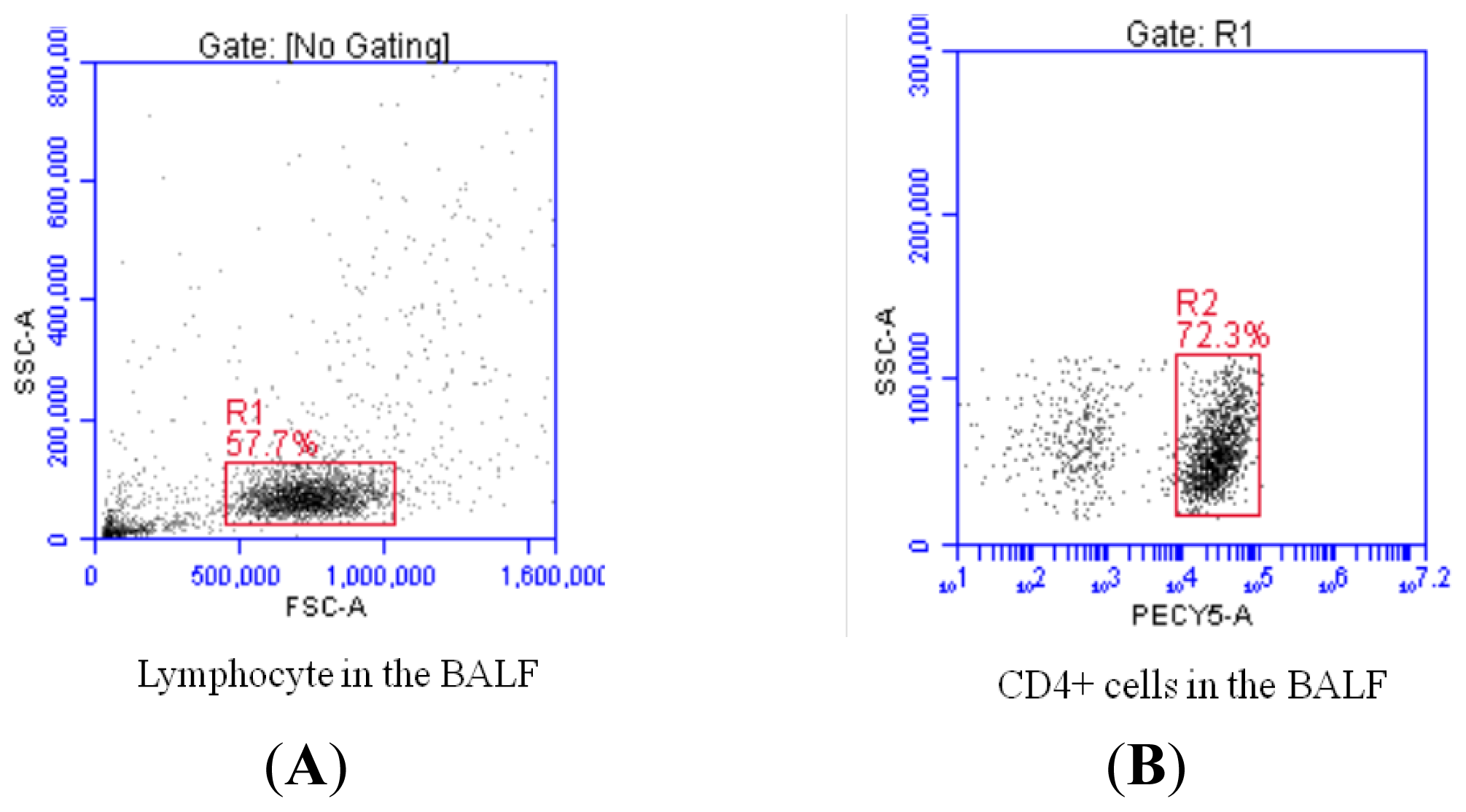
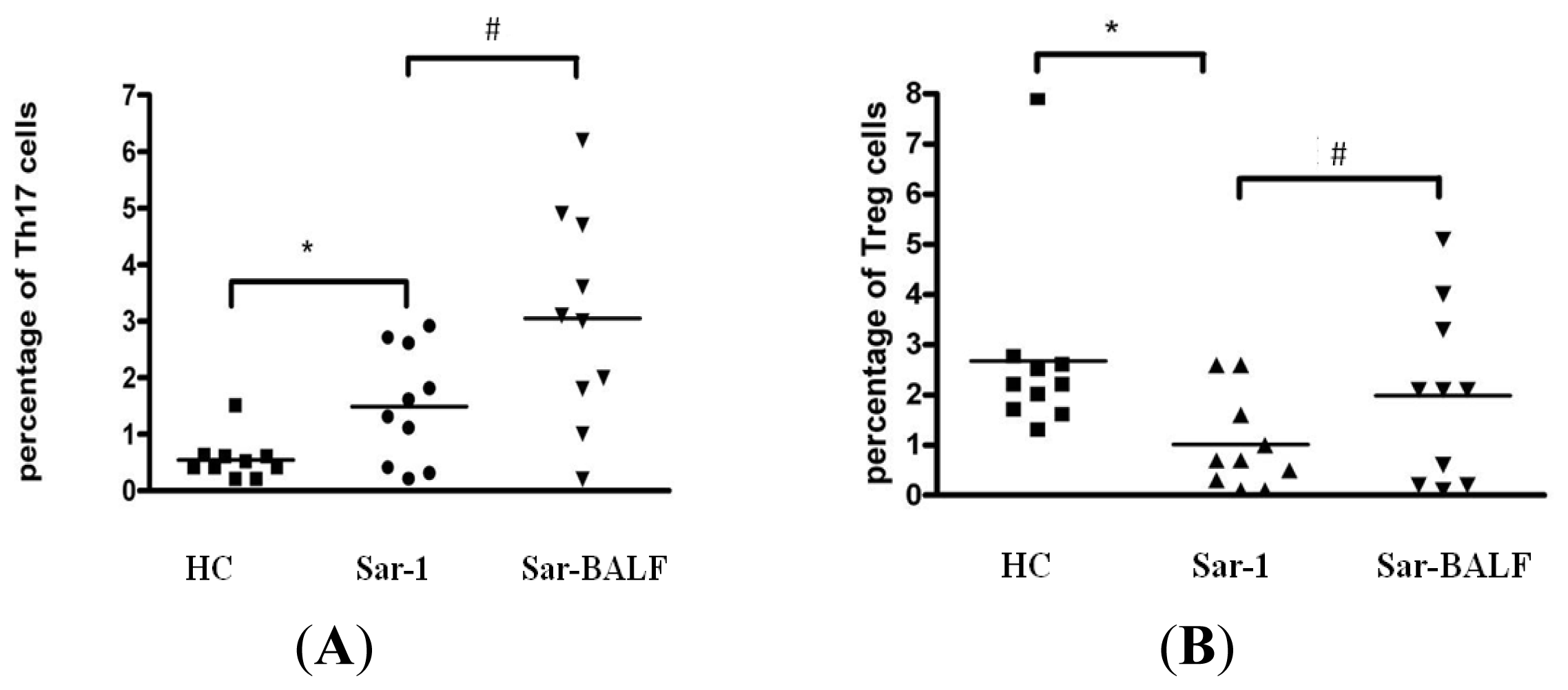
2.2. Increased Th17 Population and Decreased Treg Population in Patients with Sarcoidosis
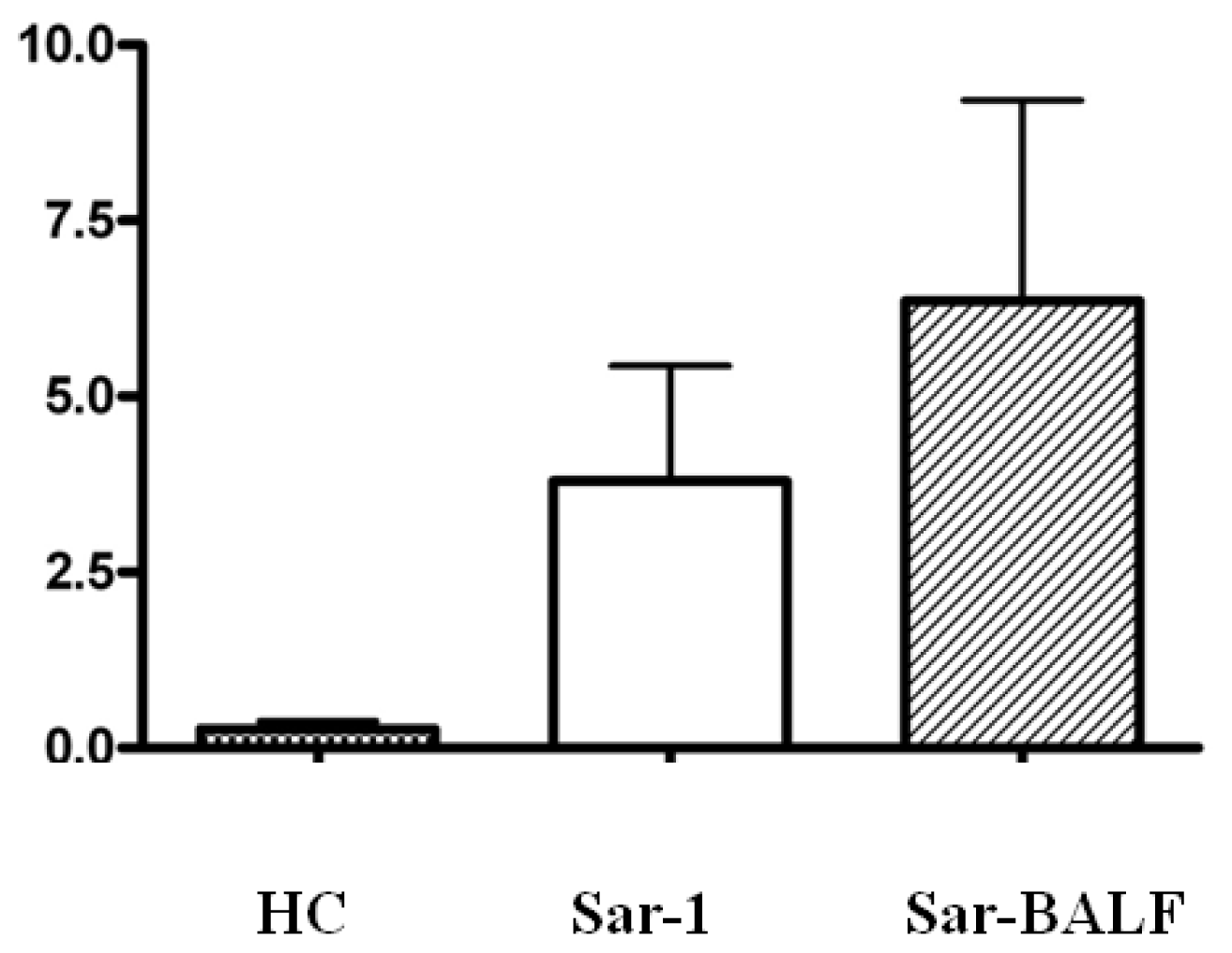
2.3. An Imbalance of Th17/Tregs in Sarcoidosis
2.4. Expression of Foxp3 and (ROR)γt in PBMCs from Sarcoidosis Patients
3. Discussion
4. Experimental Section
4.1. Patients
4.2. Cell and Sample Preparations
4.3. Flow Cytometric Analysis
4.4. Real-Time PCR Analysis
4.5. Statistical Analysis
5. Conclusions

Acknowledgments
Conflicts of Interest
References
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med 2007, 357, 2153–2165. [Google Scholar]
- Agostini, C.; Adami, F.; Semenzato, G. New pathogenetic insights into the sarcoid granuloma. Curr. Opin. Rheumatol 2000, 12, 71–76. [Google Scholar]
- Zissel, G.; Prasse, A.; Muller-Quernheim, J. Sarcoidosis—Immunopathogenetic concepts. Semin. Respir. Crit. Care Med 2007, 28, 3–14. [Google Scholar]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol 2010, 28, 445–489. [Google Scholar]
- Mai, J.; Wang, H.; Yang, X.F. Th17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front. Biosci 2010, 15, 986–1006. [Google Scholar]
- Inui, N.; Chida, K.; Suda, T.; Nakamura, H. TH1/TH2 and TC1/TC2 profiles in peripheral blood and bronchoalveolar lavage fluid cells in pulmonary sarcoidosis. J. Allergy Clin. Immunol 2001, 107, 337–344. [Google Scholar]
- Buczkowski, J.; Krawczyk, P.; Chocholska, S.; Tabarkiewicz, J.; Kieszko, R.; Michnar, M.; Milanowski, J.; Roliński, J. Blood myeloid and lymphoid dendritic cells reflect Th1/Th2 balance in sarcoidosis and extrinsic allergic alveolitis. Ann. Univ. Mariae Curie Sklodowska Med 2003, 58, 137–141. [Google Scholar]
- Nistala, K.; Wedderburn, L.R. Th17 and regulatory T cells: Rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology (Oxford) 2009, 48, 602–606. [Google Scholar]
- Zhang, L.; Yang, X.Q.; Cheng, J.; Hui, R.S.; Gao, T.W. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin. Immunol 2010, 135, 108–117. [Google Scholar]
- Eastaff-Leung, N.; Mabarrack, N.; Barbour, A.; Cummins, A.; Barry, S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol 2010, 30, 80–89. [Google Scholar]
- Ma, J.; Yu, J.; Tao, X.; Cai, L.; Wang, J.; Zheng, S.G. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin. Rheumatol 2010, 29, 1251–1258. [Google Scholar]
- Planck, A.; Katchar, K.; Eklund, A.; Gripenbäck, S.; Grunewald, J. T-lymphocyte activity in HLA-DR17 positive patients with active and clinically recovered sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis 2003, 20, 110–117. [Google Scholar]
- Miyara, M.; Amoura, Z.; Parizot, C.; Badoual, C.; Dorgham, K.; Trad, S.; Kambouchner, M.; Valeyre, D.; Chapelon-Abric, C.; Debré, P.; et al. The immune paradox of sarcoidosis and regulatory T cells. J. Exp. Med 2006, 203, 359–370. [Google Scholar]
- Rappl, G.; Pabst, S.; Riemann, D.; Schmidt, A.; Wickenhauser, C.; Schütte, W.; Hombach, A.A.; Seliger, B.; Grohé, C.; Abken, H. Regulatory T cells with reduced repressor capacities are extensively amplified in pulmonary sarcoid lesions and sustain granuloma formation. Clin. Immunol 2011, 140, 71–83. [Google Scholar]
- Mroz, R.M.; Korniluk, M.; Stasiak-Barmuta, A.; Ossolinska, M.; Chyczewska, E. Increased levels of Treg cells in bronchoalveolar lavage fluid and induced sputum of patients with active pulmonary sarcoidosis. Eur. J. Med. Res 2009, 14s, 165–169. [Google Scholar]
- Idali, F.; Wahlstrom, J.; Muller-Suur, C.; Eklund, A.; Grunewald, J. Analysis of regulatory T cell associated forkhead box P3 expression in the lungs of patients with sarcoidosis. Clin. Exp. Immunol 2008, 152, 127–137. [Google Scholar]
- Crouser, E.D.; Lozanski, G.; Fox, C.C.; Hauswirth, D.W.; Raveendran, R.; Julian, M.W. The CD4+ lymphopenic sarcoidosis phenotype is highly responsive to anti-tumor necrosis factor-{alpha} therapy. Chest 2010, 137, 1432–1435. [Google Scholar]
- Prasse, A.; Georges, C.G.; Biller, H.; Hamm, H.; Matthys, H.; Luttmann, W.; Virchow, J.C. Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin. Exp. Immunol 2000, 122, 241–248. [Google Scholar]
- Möllers, M.; Aries, S.P.; Drömann, D.; Mascher, B.; Braun, J.; Dalhoff, K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax 2001, 56, 487–493. [Google Scholar]
- Katchar, K.; Eklund, A.; Grunewald, J. Expression of Th1 markers by lung accumulated T cells in pulmonary sarcoidosis. J. Intern. Med 2003, 254, 564–571. [Google Scholar]
- Tsiligianni, I.; Antoniou, K.M.; Kyriakou, D.; Tzanakis, N.; Chrysofakis, G.; Siafakas, N.M.; Bouros, D. Th1/Th2 cytokine pattern in bronchoalveolar lavage fluid and induced sputum in pulmonary sarcoidosis. BMC Pulm. Med 2005, 5, 8. [Google Scholar] [Green Version]
- Grunewald, J.; Eklund, A.; Wahlström, J. CD4+ T cells in sarcoidosis: Targets and tools. Expert Rev. Clin. Immunol 2006, 2, 877–886. [Google Scholar]
- Xing, Q.; Wang, B.; Su, H.; Cui, J.; Li, J. Elevated Th17 cells are accompanied by FoxP3+ Treg cells decrease in patients with lupus nephritis. Rheumatol. Int 2012, 32, 949–958. [Google Scholar]
- Facco, M.; Cabrelle, A.; Teramo, A.; Olivieri, V.; Gnoato, M.; Teolato, S.; Ave, E.; Gattazzo, C.; Fadini, G.P.; Calabrese, F.; et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 2011, 66, 144–150. [Google Scholar]
- Romagnani, S. Human Th17 cells. Arthritis Res. Ther 2008, 10, 206–213. [Google Scholar]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol 2009, 27, 485–517. [Google Scholar]
- Meloni, F.; Solari, N.; Cavagna, L.; Morosini, M.; Montecucco, C.M.; Fietta, A.M. Frequency of Th1, Th2 and Th17 producing T lymphocytes in bronchoalveolar lavage of patients with systemic sclerosis. Clin. Exp. Rheumatol 2009, 27, 765–772. [Google Scholar]
- Buckner, J.H. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol 2010, 10, 849–859. [Google Scholar]
- Taflin, C.; Miyara, M.; Nochy, D.; Valeyre, D.; Naccache, J.M.; Altare, F.; Salek-Peyron, P.; Badoual, C.; Bruneval, P.; Haroche, J.; et al. FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am. J. Pathol 2009, 174, 497–508. [Google Scholar]
- Eisenstein, E.M.; Williams, C.B. The T(reg)/Th17 cell balance: A new paradigm for autoimmunity. Pediatr. Res 2009, 65, 26R–31R. [Google Scholar]
- Rong, G.; Zhou, Y.; Xiong, Y.; Zhou, L.; Geng, H.; Jiang, T.; Zhu, Y.; Lu, H.; Zhang, S.; Wang, P.; et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: The serum cytokine profile and peripheral cell population. Clin. Exp. Immunol 2009, 156, 217–225. [Google Scholar]
- Li, J.; Wang, L.; Wang, S.; Zhu, H.; Ye, P.; Xie, A.; Shen, B.; Liu, C.; Guo, C.; Fu, Q.; et al. The Treg/Th17 imbalance in patients with idiopathic dilated cardiomyopathy. Scand. J. Immunol 2010, 71, 298–303. [Google Scholar]
- Bradley, B.; Branley, H.M.; Egan, J.J.; Greaves, M.S.; Hansell, D.M.; Harrison, N.K.; Hirani, N.; Hubbard, R.; Lake, F.; Millar, A.B.; et al. Interstitial lung disease guideline: The British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008, 63s, v1–v58. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, H.; Lu, Z.; Jiang, C.; Liu, J.; Wang, Y.; Xu, Z. Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis. Int. J. Mol. Sci. 2013, 14, 21463-21473. https://doi.org/10.3390/ijms141121463
Huang H, Lu Z, Jiang C, Liu J, Wang Y, Xu Z. Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis. International Journal of Molecular Sciences. 2013; 14(11):21463-21473. https://doi.org/10.3390/ijms141121463
Chicago/Turabian StyleHuang, Hui, Zhiwei Lu, Chunguo Jiang, Jia Liu, Yanxun Wang, and Zuojun Xu. 2013. "Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis" International Journal of Molecular Sciences 14, no. 11: 21463-21473. https://doi.org/10.3390/ijms141121463



