Short-Chain Fatty Acids Inhibit Growth Hormone and Prolactin Gene Transcription via cAMP/PKA/CREB Signaling Pathway in Dairy Cow Anterior Pituitary Cells
Abstract
:1. Introduction
2. Results
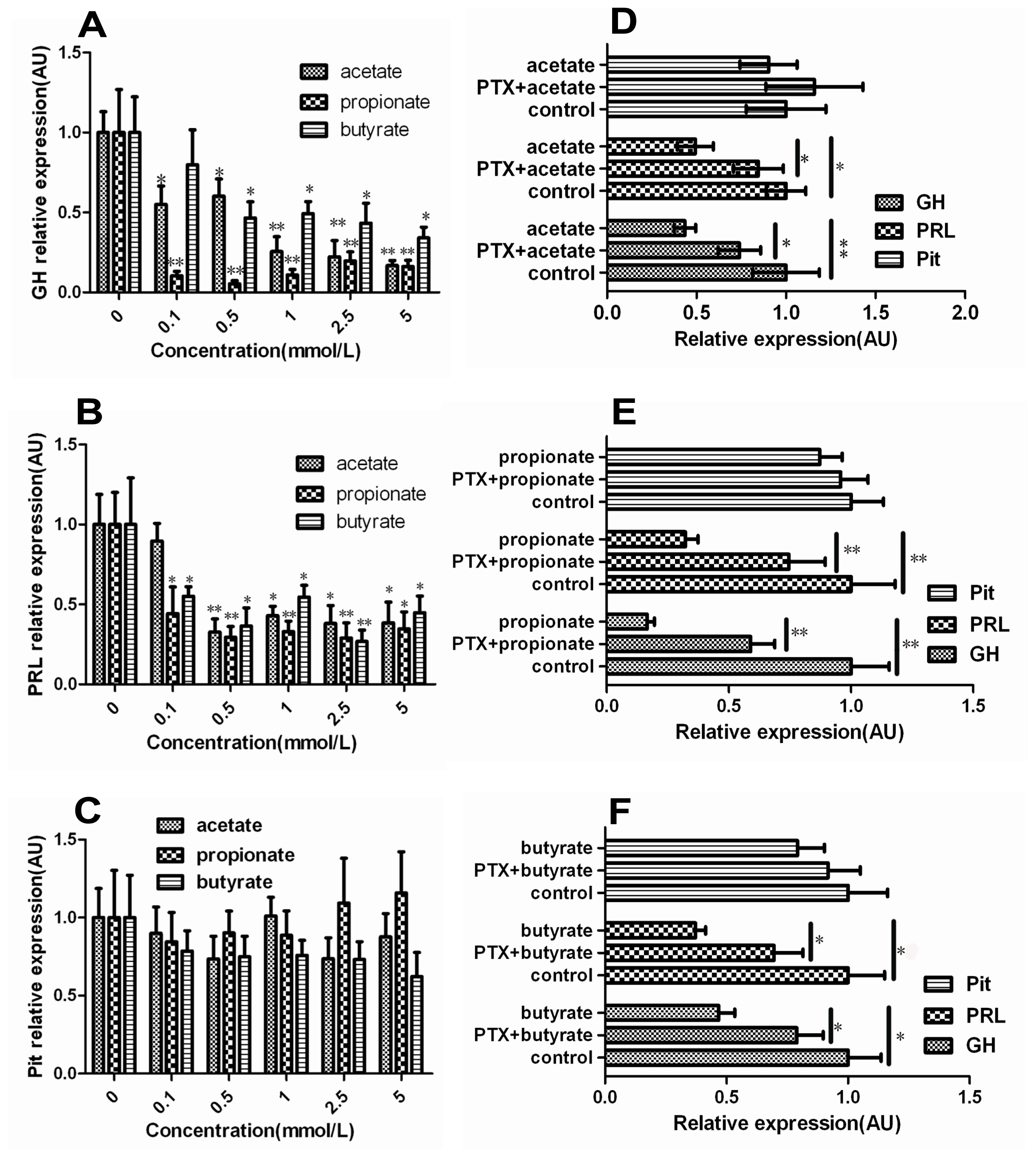
2.1. Effect of SCFAs on mRNA Levels of GH, PRL and Pit-1 in DCAPCs
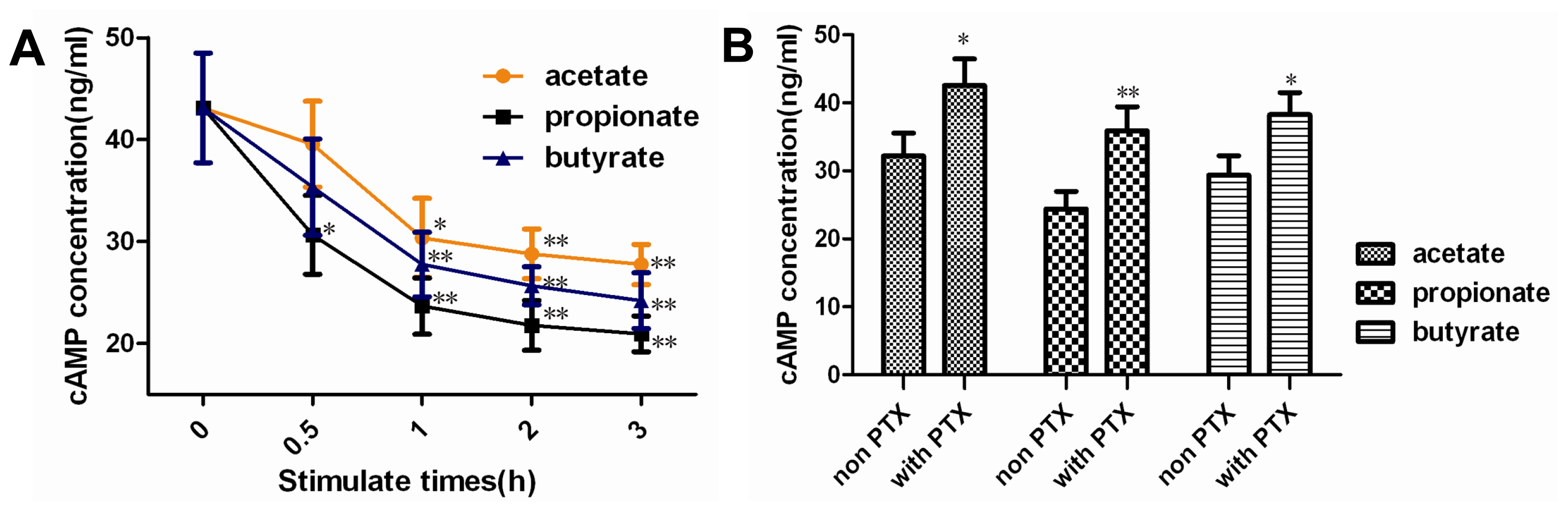
2.2. Effect of SCFAs on Intracellular cAMP Concentration
2.3. Effect of SCFAs on PKA Activity
2.4. Effect of SCFAs on CREB Phosphorylation
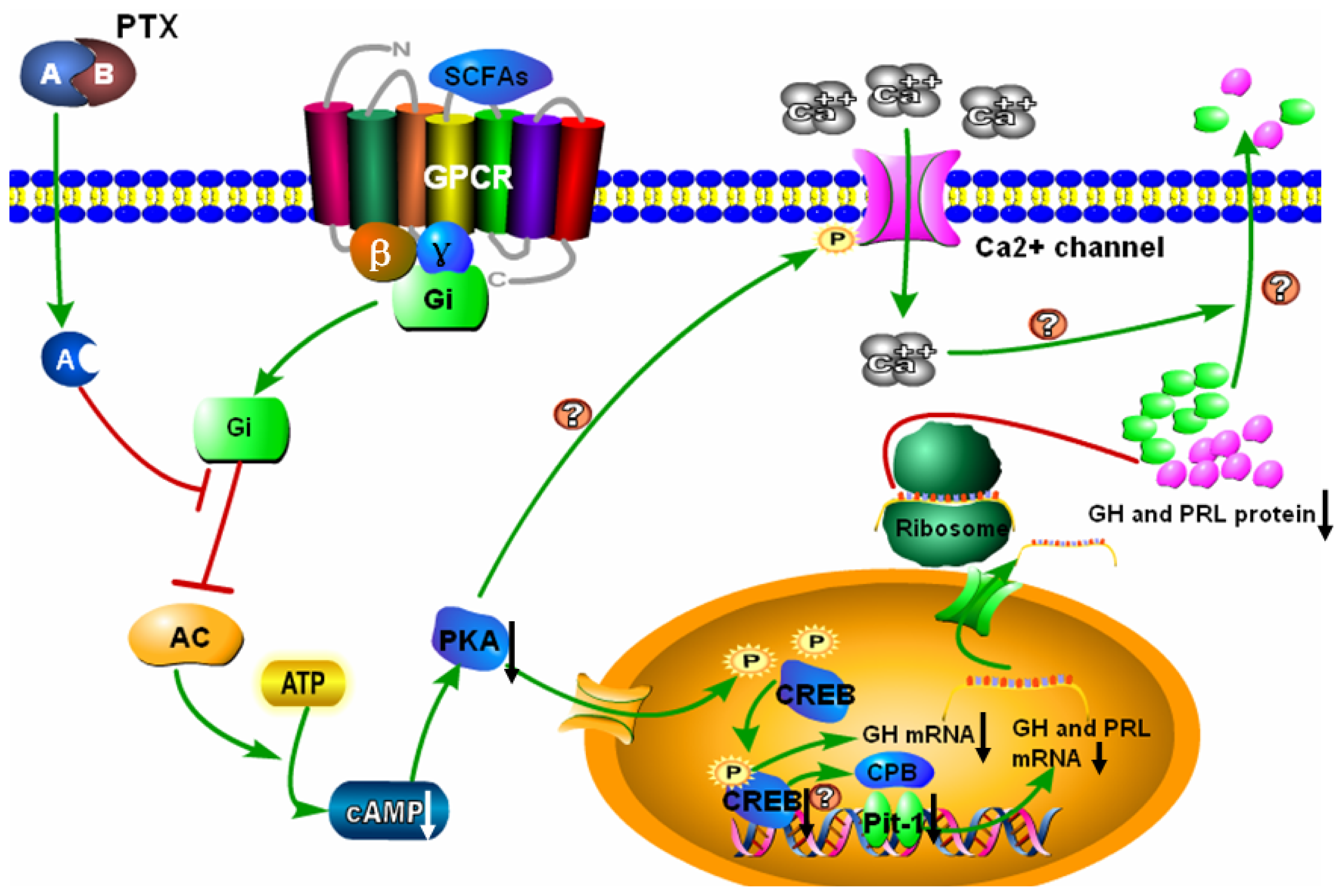
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Isolation and Culture of DCAPCs
4.3. Real-Time RT-PCR
4.4. Measurement of Intracellular cAMP Concentration
4.5. Measurement of PKA Activity
4.6. Western Blotting Analysis of Phosphorylated CREB
4.7. Statistical Analyses
5. Conclusions


| Gene | Sequences | Length (bp) |
|---|---|---|
| GAPDH | (F)5′-TGCCCAGAATATCATCCC-3′ (R)5′-AGGTCAGATCCACAACAG-3′ | 134 |
| GH | (F)5′-AGATCCTCAAGCAGACCTA-3′ (R)5′-AGGTACGTCTCCGTCTTA-3′ | 121 |
| PRL | (F)5′-TATGAAAGGAGCCCCAGATG-3′ (R)5′-CACACAGGGTAGGGCTCAGT-3′ | 137 |
| Pit-1 | (F)5′-TTCTGCAACTCTGCCTCTGA-3′ (R)5′-CCATAGGTCGATGACTGGT-3′ | 148 |
Acknowledgments
Conflicts of Interest
References
- Ooi, G.T.; Tawadros, N.; Escalona, R.M. Pituitary cell lines and their endocrine applications. Mol. Cell Biol 2004, 228, 1–21. [Google Scholar]
- Akers, R.M. Major advances associated with hormone and growth factor regulation of mammary growth and lactation in dairy cows. J. Dairy Sci 2006, 89, 1222–1234. [Google Scholar]
- Capuco, A.V.; Wood, D.L.; Baldwin, R.; McLeod, K.; Paape, M.J. Mammary cell number, proliferation, and apoptosis during a bovine lactation: Relation to milk production and effect of bst. J. Dairy Sci 2001, 84, 2177–2187. [Google Scholar]
- Yang, J.; Zhao, B.; Baracos, V.E.; Kennelly, J.J. Effects of bovine somatotropin on β-casein mrna levels in mammary tissue of lactating cows. J. Dairy Sci 2005, 88, 2806–2812. [Google Scholar]
- Yonekura, S.; Sakamoto, K.; Komatsu, T.; Hagino, A.; Katoh, K.; Obara, Y. Growth hormone and lactogenic hormones can reduce the leptin mRNA expression in bovine mammary epithelial cells. Domest. Anim. Endocrinol 2006, 31, 88–96. [Google Scholar]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev 2000, 4, 1523–1631. [Google Scholar]
- Trott, J.F.; Schennink, A.; Petrie, W.K.; Manjarin, R.; VanKlompenberg, M.K.; Hovey, R.C. Triennial lactation symposium: Prolactin: The multifaceted potentiator of mammary growth and function. J. Anim. Sci 2012, 95, 1674–1686. [Google Scholar]
- Wong, J.M.; Souza, R.; Kendall, C.W.; Dumas, M.E. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol 2006, 40, 235–243. [Google Scholar]
- Sutton, J.D.; Dhanoa, M.S.; Morant, S.V.; France, J.; Napper, D.J.; Schuller, E. Rates of production of acetate, propionate, and butyrate in the rumen of lactating dairy cows given normal and low-roughage diets. J. Dairy Sci 2003, 86, 3620–3633. [Google Scholar]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar]
- Blottiere, H.M.; Buecher, B.; Galmiche, J.P.; Cherbut, C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc. Nutr. Soc 2003, 62, 101–106. [Google Scholar]
- Mineo, H.; Hashizume, Y.; Hanaki, Y.; Murata, K.; Maeda, H.; Onaga, T.; Kato, S.; Yanaihara, N. Chemical specificity of short-chain fatty acids in stimulating insulin and glucagon secretion in sheep. Am. J. Physiol 1994, 267, E234–E241. [Google Scholar]
- Kato, S.; Sato, K.; Chida, H.; Roh, S.G.; Ohwada, S.; Sato, S.; Guilloteau, P.; Katoh, K. Effects of Na-butyrate supplementation in milk formula on plasma concentrations of GH and insulin, and on rumen papilla development in calves. J. Endocrinol 2011, 211, 241–248. [Google Scholar]
- Yen, P.M.; Tashjian, A.H., Jr. Short chain fatty acids increase prolactin and growth hormone production and alter cell morphology in the GH3 strain of rat pituitary cells. Endocrinology 1981, 109, 17–22. [Google Scholar]
- Ishiwata, H.; Nagano, M.; Sasaki, Y.; Chen, C.; Katoh, K. Short-chain fatty acids inhibit the release and content of growth hormone in anterior pituitary cells of the goat. Gen. Comp. Endocrinol 2000, 118, 400–406. [Google Scholar]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem 2003, 278, 11312–11319. [Google Scholar]
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.I.; Kuwahara, A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol 2008, 59, 251–262. [Google Scholar]
- Wang, A.; Gu, Z.; Heid, B.; Akers, R.M.; Jiang, H. Identification and characterization of the bovine G protein-coupled receptor GPR4 1 and GPR43 genes. J. Dairy Sci 2009, 92, 2696–2705. [Google Scholar]
- Nelson, C.; Albert, V.R.; Elsholtz, H.P.; Lu, L.I.; Rosenfeld, M.G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science 1988, 239, 1400–1405. [Google Scholar]
- Sanchez-Pacheco, A.; Palomino, T.; Aranda, A. Negative regulation of expression of the pituitary-specific transcription factor GHF-1/Pit-1 by thyroid hormones through interference with promoter enhancer elements. Mol. Cell Biol 1995, 15, 6322–6330. [Google Scholar]
- Schaufele, F.; West, B.L.; Reudelhuber, T.L. Overlapping Pit-1 and Sp1 binding sites are both essential to full rat growth hormone gene promoter activity despite mutually exclusive Pit-1 and Sp1 binding. J. Biol. Chem 1990, 265, 17189–17196. [Google Scholar]
- Gaiddon, C.; Tian, J.; Loeffler, J.P.; Bancroft, C. Constitutively active G(S) α-subunits stimulate Pit-1 promoter activity via a protein kinase A-mediated pathway acting through deoxyribonucleic acid binding sites both for Pit-1 and for adenosine 3′,5′-monophosphate response element-binding protein. Endocrinology 1996, 137, 1286–1291. [Google Scholar]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev 2001, 81, 1031–1064. [Google Scholar]
- Den Hond, E.; Hiele, M.; Evenepoel, P.; Peeters, M.; Ghoos, Y.; Rutgeerts, P. In vivo butyrate metabolism and colonic permeability in extensive ulcerative colitis. Gastroenterology 1998, 115, 584–590. [Google Scholar]
- Annison, G.; Illman, R.J.; Topping, D.L. Acetylated, propionylated or butyrylated starches raise large bowel short-chain fatty acids preferentially when fed to rats. J. Nutr 2003, 133, 3523–3528. [Google Scholar]
- Sauer, J.; Richter, K.K.; Pool-Zobel, B.L. Products formed during fermentation of the prebiotic inulin with humangut flora enhance expression of biotranformation genes in human primary colon cells. Br. J. Nutr 2007, 97, 928–937. [Google Scholar]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther 2008, 27, 104–119. [Google Scholar]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic micro- biota: Introducing the concept of prebiotics. J. Nutr 1995, 125, 1401–1412. [Google Scholar]
- Pan, X.D.; Chen, F.Q.; Wu, T.X.; Tang, H.G.; Zhao, Z.Y. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J. Zhejiang Univ. Sci. B 2009, 10, 258–263. [Google Scholar]
- Thomsen, W.; Frazer, J.; Unett, D. Functional assays for screening GPCR targets. Curr. Opin. Biotechnol 2005, 16, 655–665. [Google Scholar]
- El-Armouche, A.; Zolk, O.; Rau, T.; Eschenhagen, T. Inhibitory G-proteins and their role in desensitization of the adenylyl cyclase pathway in heart failure. Cardiovasc. Res 2003, 60, 478–487. [Google Scholar]
- Mangmool, S.; Kurose, H. Gi/o protein-dependent and -independent actions of pertussis toxin (PTX). Toxins 2011, 3, 884–899. [Google Scholar]
- Tian, C.; Ye, F.; Xu, T.; Wang, S.; Wang, X.; Wang, H.; Wan, F.; Lei, T. GHRP-6 induces CREB phosphorylation and growth hormone secretion via a protein kinase Cσ-dependent pathway in GH3 cells. J. Huazhong Univ. Sci. Technol 2010, 30, 183–187. [Google Scholar]
- Jean, A.; Gutierrez-Hartmann, A.; Duval, D.L. A Pit-1 threonine 220 phosphomimic reduces binding to monomeric DNA sites to inhibit Ras and estrogen stimulation of the prolactin gene promoter. Mol. Endocrinol 2010, 24, 91–103. [Google Scholar]
- Kapiloff, M.S.; Farkash, Y.; Wegner, M.; Rosenfeld, M.G. Variable effects of phosphorylation of Pit-1 dictated by the DNA response elements. Science 1991, 253, 786–789. [Google Scholar]
- Caelles, C.; Hennemann, H.; Karin, M. M-phase-specific phosphorylation of the POU transcription factor GHF-1 by a cell cycle-regulated protein kinase inhibits DNA binding. Mol. Cell Biol 1995, 15, 6694–6701. [Google Scholar]
- Cohen, L.E.; Hashimoto, Y.; Zanger, K.; Wondisford, F.; Radovick, S. CREB-independent regulation by CBP is a novel mechanism of human growth hormone gene expression. J. Clin. Invest 1999, 104, 1123–1130. [Google Scholar]
- Lundblad, J.R.; Kwok, R.P.S.; Laurance, M.E.; Harter, M.L.; Goodman, R.H. Adenoviral E1A-associated p300 as a functional homologue of the transcriptional co-activator CBP. Nature 1995, 374, 85–88. [Google Scholar]
- Lane, M.A.; Jesse, B.W. Effect of volatile fatty acid infusion on development of the rumen epithelium in neonatal sheep. J. Dairy Sci 1997, 80, 740–746. [Google Scholar]
- Zhao, G.Y.; Sun, Y.B. Effects of volatile fatty acids on IGF-I, IGFBP-3, GH, insulin and glucagon in plasma, and IGF-I and IGFBP-3 in different tissues of growing sheep nourished by total intragastric infusions. Asian-Aust. J. Anim. Sci 2010, 23, 366–371. [Google Scholar]
- Matsunaga, N.; Kubota, I.; Roh, S.G.; He, M.L.; Hidaka, S.; Hidari, H.; Sasaki, Y. Effect of mesenteric venous volatile fatty acids (VFA) infusion on GH secretion in sheep. Endocr. J 1997, 44, 707–714. [Google Scholar]
- Matsunaga, N.; Arakawa, N.T.; Goka, T.; Nam, K.T.; Ohneda, A.; Sasaki, Y.; Katoh, K. Effects of ruminal infusion of volatile fatty acids on plasma concentration of growth hormone and insulin in sheep. Domest. Anim. Endocrinol 1999, 17, 17–27. [Google Scholar]
- Matsunaga, N.; Goka, T.; Nam, K.T.; Oda, S.; Ohneda, A.; Sasaki, Y. Inhibition of GH releasing factor (GRF)-induced GH secretion by intraruminal infusion of volatile fatty acids (VFA) in sheep. Endocr. J 1997, 44, 133–140. [Google Scholar]
- Katoh, K.; Takahashi, T.; Kobayashi, Y.; Obara, Y. Somatotropic axis and nutrition in young ruminants around weaning time. Asian-Aust. J. Anim. Sci 2007, 20, 1156–1168. [Google Scholar]
- Wang, J.F.; Fu, S.P.; Li, S.N.; Yang, Z.Q.; Xue, W.J.; Li, Z.Q.; Wang, W.; Liu, J.X. Establishment and characterization of dairy cow growth hormone secreting anterior pituitary cell model. In Vitro Cell Dev. Biol. Anim 2013, in press. [Google Scholar]
- Sato, H.; Kurosawa, T.; Oikawa, S. Acetic acid and 3-hydroxybutyric acid levels in cerebrospinal fluid of cattle. Anim. Sci. J 2002, 73, 137–141. [Google Scholar]
- Laeger, T.; Sauerwein, H.; Tuchscherer, A.; Bellmann, O.; Metges, C.C.; Kuhla, B. Concentrations of hormones and metabolites in cerebrospinal fluid and plasma of dairy cows during the periparturient period. J. Dairy Sci 2013, 96, 2883–2893. [Google Scholar]
- Bruckener, K.E.; el Baya, A.; Galla, H.J.; Schmidt, M.A. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J. Cell Sci 2003, 116, 1837–1846. [Google Scholar]
- Laporta, J.; Driver, A.; Khatib, H. Short communication: Expression and alternative splicing of POU1F1 pathway genes in preimplantation bovine embryos. J. Dairy Sci 2011, 94, 4220–4223. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 2001, 25, 402–408. [Google Scholar]
- Lisowski, P.; Pierzcha, M.; Gooecik, J. Evaluation of reference genes for studies of gene expression in the bovine liver, kidney, pituitary, and thyroid. J. Appl. Genet 2008, 49, 367–372. [Google Scholar]
- Wehrens, X.H.; Lehnart, S.E.; Reiken, S.; Vest, J.A.; Wronska, A.; Marks, A.R. Ryanodine receptor/calcium release channel PKA phosphorylation: A critical mediator of heart failure progression. Proc. Natl. Acad. Sci. USA 2006, 103, 511–518. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.-F.; Fu, S.-P.; Li, S.-N.; Hu, Z.-M.; Xue, W.-J.; Li, Z.-Q.; Huang, B.-X.; Lv, Q.-K.; Liu, J.-X.; Wang, W. Short-Chain Fatty Acids Inhibit Growth Hormone and Prolactin Gene Transcription via cAMP/PKA/CREB Signaling Pathway in Dairy Cow Anterior Pituitary Cells. Int. J. Mol. Sci. 2013, 14, 21474-21488. https://doi.org/10.3390/ijms141121474
Wang J-F, Fu S-P, Li S-N, Hu Z-M, Xue W-J, Li Z-Q, Huang B-X, Lv Q-K, Liu J-X, Wang W. Short-Chain Fatty Acids Inhibit Growth Hormone and Prolactin Gene Transcription via cAMP/PKA/CREB Signaling Pathway in Dairy Cow Anterior Pituitary Cells. International Journal of Molecular Sciences. 2013; 14(11):21474-21488. https://doi.org/10.3390/ijms141121474
Chicago/Turabian StyleWang, Jian-Fa, Shou-Peng Fu, Su-Nan Li, Zhong-Ming Hu, Wen-Jing Xue, Zhi-Qiang Li, Bing-Xu Huang, Qing-Kang Lv, Ju-Xiong Liu, and Wei Wang. 2013. "Short-Chain Fatty Acids Inhibit Growth Hormone and Prolactin Gene Transcription via cAMP/PKA/CREB Signaling Pathway in Dairy Cow Anterior Pituitary Cells" International Journal of Molecular Sciences 14, no. 11: 21474-21488. https://doi.org/10.3390/ijms141121474




