Combination of Fenretinide and Selenite Inhibits Proliferation and Induces Apoptosis in Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Fenretinide and Selenite Enhance Suppression of Proliferation of Ovarian Cancer Cell Lines
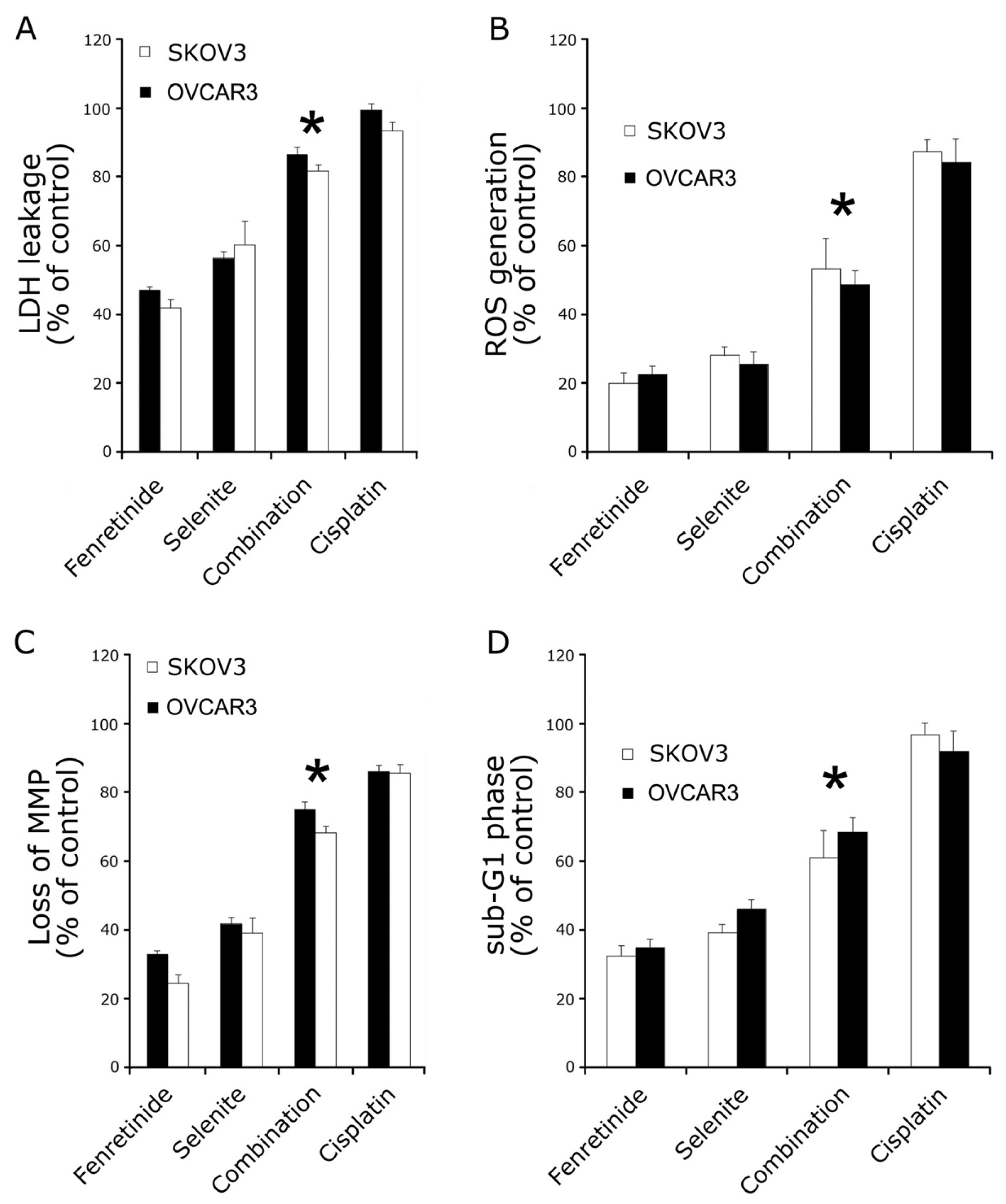
2.2. Fenretinide and Selenite Induce Ovarian Cancer Cell Lines Apoptosis
2.3. AMPK Mediated Fenretinide and Selenite Combination-Induced Apoptosis
2.4. Fenretinide and Selenite Combination on Tumor Growth in SKOV3 Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Cell Culture and Reagents
4.2. Cell Viability Assay
4.3. Lactate Dehydrogenase (LDH) Leakage Assay
4.4. Intracellular Reactive Oxygen Species (ROS) Measurement
4.5. Propidium Iodide (PI) Staining and Flow Cytometry Analysis
4.6. Mitochondrial Membrane Potential Assays
4.7. Assay of Caspase Activity
4.8. AMPK Activation Assay
4.9. Subcellular Fraction Isolation
4.10. Western Blotting
4.11. Ovarian Cancer Cell Xenograft Models
4.12. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-14-21790-s001.pdf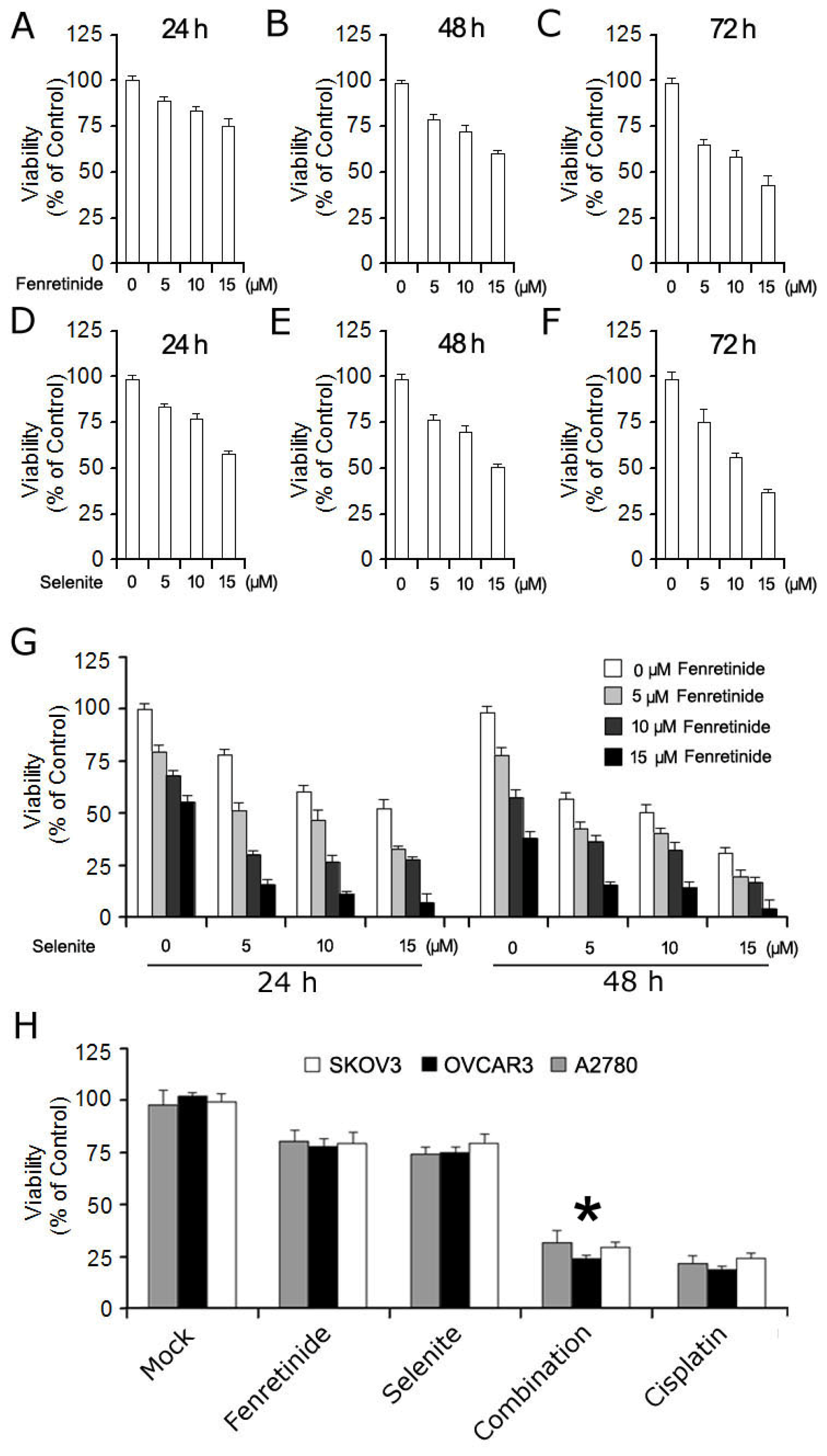



Conflicts of Interest
References
- Assis, J.; Pereira, D.; Medeiros, R. Ovarian cancer and DNA repair: DNA ligase IV as a potential key. World J. Clin. Oncol 2013, 4, 14–24. [Google Scholar]
- Hojka-Osinska, A.; Ziolo, E.; Rapak, A. Combined treatment with fenretinide and indomethacin induces AIF-mediated, non-classical cell death in human acute T-cell leukemia Jurkat cells. Biochem. Biophys. Res. Commun 2012, 419, 590–595. [Google Scholar]
- Armstrong, J.L.; Martin, S.; Illingworth, N.A.; Jamieson, D.; Neilson, A.; Lovat, P.E.; Redfern, C.P.; Veal, G.J. The impact of retinoic acid treatment on the sensitivity of neuroblastoma cells to fenretinide. Oncol. Rep 2012, 27, 293–298. [Google Scholar]
- Kim, K.K.; Lange, T.S.; Singh, R.K.; Brard, L.; Moore, R.G. Tetrathiomolybdate sensitizes ovarian cancer cells to anticancer drugs doxorubicin, fenretinide, 5-fluorouracil and mitomycin C. BMC Cancer 2012. [Google Scholar] [CrossRef]
- Holpuch, A.S.; Phelps, M.P.; Desai, K.G.; Chen, W.; Koutras, G.M.; Han, B.B.; Warner, B.M.; Pei, P.; Seghi, G.A.; Tong, M.; et al. Evaluation of a mucoadhesive fenretinide patch for local intraoral delivery: A strategy to reintroduce fenretinide for oral cancer chemoprevention. Carcinogenesis 2012, 33, 1098–1105. [Google Scholar]
- Pagnan, G.; Di-Paolo, D.; Carosio, R.; Pastorino, F.; Marimpietri, D.; Brignole, C.; Pezzolo, A.; Loi, M.; Galietta, L.J.; Piccardi, F.; et al. The combined therapeutic effects of bortezomib and fenretinide on neuroblastoma cells involve endoplasmic reticulum stress response. Clin. Cancer Res 2009, 15, 1199–1209. [Google Scholar]
- Zhang, H.; Mi, J.Q.; Fang, H.; Wang, Z.; Wang, C.; Wu, L.; Zhang, B.; Minden, M.; Yang, W.T.; Wang, H.W.; et al. Preferential eradication of acute myelogenous leukemia stem cells by fenretinide. Proc. Natl. Acad. Sci. USA 2013, 110, 5606–5611. [Google Scholar]
- Yasuo, M.; Mizuno, S.; Allegood, J.; Kraskauskas, D.; Bogaard, H.J.; Spiegel, S.; Voelkel, N.F. Fenretinide causes emphysema, which is prevented by sphingosine 1-phoshate. PLoS One 2013, 8, e53927. [Google Scholar]
- Kadara, H.; Tahara, E.; Kim, H.J.; Lotan, D.; Myers, J.; Lotan, R. Involvement of Rac in fenretinide-induced apoptosis. Cancer Res 2008, 68, 4416–4423. [Google Scholar]
- Ardekani, A.M.; Fard, S.S.; Jeddi-Tehrani, M.; Ghahremanzade, R. Bryostatin-1, fenretinide and 1α,25 (OH)2D3 induce growth inhibition, apoptosis and differentiation in T and B Cell-derived acute lymphoblastic leukemia cell lines (CCRF-CEM and Nalm-6). Avicenna J. Med. Biotechnol 2011, 3, 177–193. [Google Scholar]
- Yang, H.; Bushue, N.; Bu, P.; Wan, Y.J. Inductionand intracellular localization of Nur77 dictate fenretinide-induced apoptosis of human liver cancer cells. Biochem. Pharmacol 2010, 79, 948–954. [Google Scholar]
- Raguénez, G.; Mühlethaler-Mottet, A.; Meier, R.; Duros, C.; Bénard, J.; Gross, N. Fenretinide-induced caspase-8 activation and apoptosis in an established model of metastatic neuroblastoma. BMC Cancer 2009. [Google Scholar] [CrossRef]
- Fang, H.; Harned, T.M.; Kalous, O.; Maldonado, V.; DeClerck, Y.A.; Reynolds, C.P. Synergistic activity of fenretinide and the Bcl-2 family protein inhibitor ABT-737 against human neuroblastoma. Clin. Cancer Res 2011, 17, 7093–7104. [Google Scholar]
- Holmes, W.F.; Soprano, D.R.; Soprano, K.J. Comparison of the mechanism of induction of apoptosis in ovarian carcinoma cells by the conformationally restricted synthetic retinoids CD437 and 4-HPR. J. Cell. Biochem 2003, 89, 262–278. [Google Scholar]
- Yang, H.; Zhan, Q.; Wan, Y.J. Enrichment of Nur77 mediated by retinoic acid receptor β leads to apoptosis of human hepatocellular carcinoma cells induced by fenretinide and histone deacetylase inhibitors. Hepatology 2011, 53, 865–874. [Google Scholar]
- White, D.E.; Burchill, S.A. Fenretinide-dependent upregulation of death receptors through ASK1 and p38α enhances death receptor ligand-induced cell death in Ewing’s sarcoma family of tumours. Br. J. Cancer 2010, 103, 1380–1390. [Google Scholar]
- Koay, D.C.; Zerillo, C.; Narayan, M.; Harris, L.N.; DiGiovanna, M.P. Anti-tumor effects of retinoids combined with trastuzumab or tamoxifen in breast cancer cells: Induction of apoptosis by retinoid/trastuzumab combinations. Breast Cancer Res 2010. [Google Scholar] [CrossRef]
- Cuperus, R.; Leen, R.; Tytgat, G.A.; Caron, H.N.; van Kuilenburg, A.B. Fenretinide induces mitochondrial ROS and inhibits the mitochondrial respiratory chain in neuroblastoma. Cell. Mol. Life Sci 2010, 67, 807–816. [Google Scholar]
- Park, S.H.; Kim, J.H.; Chi, G.Y.; Kim, G.Y.; Chang, Y.C.; Moon, S.K.; Nam, S.W.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol. Lett 2012, 212, 252–261. [Google Scholar]
- Gowda, R.; Madhunapantula, S.V.; Desai, D.; Amin, S.; Robertson, G.P. Selenium-containing histone deacetylase inhibitors for melanoma management. Cancer Biol. Ther 2012, 13, 756–765. [Google Scholar]
- Kim, Y.W.; Bae, S.M.; Liu, H.B.; Kim, I.W.; Chun, H.J.; Ahn, W.S. Selenium enhances the efficacy of Radachlorin mediated-photodynamic therapy in TC-1 tumor development. Oncol. Rep 2012, 28, 576–584. [Google Scholar]
- Lei, M.; Chen, D.; Deng, X.; Liu, J.; Chen, L.; Liu, Y.; Li, B.; Yao, H.; Xiong, G.; Cao, Y.; et al. Dynamic sieving capillary electrophoresis analysis of xylitol selenite-induced apoptosis in SMMC-7221 cells. Biotechnol. Lett 2012, 34, 1617–1621. [Google Scholar]
- Huang, C.; Ding, G.; Gu, C.; Zhou, J.; Kuang, M.; Ji, Y.; He, Y.; Kondo, T.; Fan, J. Decreased selenium-binding protein 1 enhances glutathione peroxidase 1 activity and downregulates HIF-1α to promote hepatocellular carcinoma invasiveness. Clin. Cancer Res 2012, 18, 3042–3053. [Google Scholar]
- Yang, J.; Yang, X.; Fan, J.; Zhao, Q.; Xu, C. The novel selenium heteropoly compound (NH4)4H4[Se2Mo2V4O24]·7H2O induces apoptosis of K562 cells. Mol. Med. Rep 2011, 4, 1327–1332. [Google Scholar]
- Zhu, X.; Guo, K.; Lu, Y. Selenium effectively inhibits 1,2-dihydroxynaphthalene-induced apoptosis in human lens epithelial cells through activation of PI3-K/Akt pathway. Mol. Vis 2011, 17, 2019–2027. [Google Scholar]
- Cassidy, P.B.; Fain, H.D.; Cassidy, J.P.; Tran, S.M.; Moos, P.J.; Boucher, K.M.; Gerads, R.; Florell, S.R.; Grossman, D.; Leachman, S.A. Selenium for the prevention of cutaneous melanoma. Nutrients 2013, 5, 725–749. [Google Scholar]
- Kim, S.L.; Kim, S.H.; Trang, K.T.; Kim, I.H.; Lee, S.O.; Lee, S.T.; Kim, D.G.; Kang, S.B.; Kim, S.W. Synergistic antitumor effect of 5-fluorouracil in combination with parthenolide in human colorectal cancer. Cancer Lett 2013, 335, 479–486. [Google Scholar]
- Holmes, W.F.; Soprano, D.R.; Soprano, K.J. Synthetic retinoids as inducers of apoptosis in ovarian carcinoma cell lines. J. Cell. Physiol 2004, 199, 317–329. [Google Scholar]
- Chen, L.; Han, F.; Qu, H.; Yan, H.; Ren, L.; Yang, S. Combination therapy with 5-amino-4-imidazolecarboxamide riboside and arsenic trioxide in acute myeloid leukemia cells involving AMPK/TSC2/mTOR pathway. Pharmazie 2013, 68, 117–123. [Google Scholar]
- Luo, H.; Yang, Y.; Duan, J.; Wu, P.; Jiang, Q.; Xu, C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis 2013. [Google Scholar] [CrossRef]
- He, N.; Shi, X.; Zhao, Y.; Tian, L.; Wang, D.; Yang, X. Inhibitory effects and molecular mechanisms of selenium-containing tea polysaccharides on human breast cancer MCF-7 cells. J. Agric. Food Chem 2013, 61, 579–588. [Google Scholar]
- Selvaraj, V.; Tomblin, J.; Yeager Armistead, M.; Murray, E. Selenium (sodium selenite) causes cytotoxicity and apoptotic mediated cell death in PLHC-1 fish cell line through DNA and mitochondrial membrane potential damage. Ecotoxicol. Environ. Saf 2013, 87, 80–88. [Google Scholar]
- Selvaraj, V.; Yeager-Armstead, M.; Murray, E. Protective and antioxidant role of selenium on arsenic trioxide-induced oxidative stress and genotoxicity in the fish hepatoma cell line PLHC-1. Environ. Toxicol. Chem 2012, 31, 2861–2869. [Google Scholar]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci 2012, 13, 9649–9572. [Google Scholar]
- Zheng, S.; Li, X.; Zhang, Y.; Xie, Q.; Wong, Y.S.; Zheng, W.; Chen, T. PEG-nanolized ultrasmall selenium nanoparticles overcome drug resistance in hepatocellular carcinoma HepG2 cells through induction of mitochondria dysfunction. Int. J. Nanomed 2012, 7, 3939–3949. [Google Scholar]
- Liu, W.; Li, X.; Wong, Y.S.; Zheng, W.; Zhang, Y.; Cao, W.; Chen, T. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS Nano 2012, 6, 6578–6591. [Google Scholar]
- Kong, L.; Yuan, Q.; Zhu, H.; Li, Y.; Guo, Q.; Wang, Q.; Bi, X.; Gao, X. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials 2011, 32, 6515–6522. [Google Scholar]
- Shang, D.; Li, Y.; Wang, C.; Wang, X.; Yu, Z.; Fu, X. A novel polysaccharide from Se-enriched Ganoderma lucidum induces apoptosis of human breast cancer cells. Oncol. Rep 2011, 25, 267–272. [Google Scholar]
- Wu, M.; Kang, M.M.; Schoene, N.W.; Cheng, W.H. Selenium compounds activate early barriers of tumorigenesis. J. Biol. Chem 2010, 285, 12055–12062. [Google Scholar]
- Abdulah, R.; Faried, A.; Kobayashi, K.; Yamazaki, C.; Suradji, E.W.; Ito, K.; Suzuki, K.; Murakami, M.; Kuwano, H.; Koyama, H. Selenium enrichment of broccoli sprout extract increases chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC Cancer 2009. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Naziroğlu, M.; Espino, J.; Bejarano, I.; González, D.; Rodríguez, A.B.; Pariente, J.A. Selenium modulates oxidative stress-induced cell apoptosis in human myeloid HL-60 cells through regulation of calcium release and caspase-3 and -9 activities. J. Membr. Biol 2009, 232, 15–23. [Google Scholar]
- Das, A.; Bortner, J.; Desai, D.; Amin, S.; El-Bayoumy, K. The selenium analog of the chemopreventive compound S,S′-[1,4-phenylenebis(1,2-ethanediyl)] bisisothiourea is a remarkable inducer of apoptosis and inhibitor of cell growth in human non-small cell lung cancer. Chem. Biol. Interact 2009, 180, 158–164. [Google Scholar]
- Huang, F.; Nie, C.; Yang, Y.; Yue, W.; Ren, Y.; Shang, Y.; Wang, X.; Jin, H.; Xu, C.; Chen, Q. Selenite induces redox-dependent Bax activation and apoptosis in colorectal cancer cells. Free Radic. Biol. Med 2009, 46, 1186–1196. [Google Scholar]
- Yang, J.Y.; Xu, C.S. Antitumor effects of a selenium heteropoly complex in K562 cells. Pharmacol. Rep 2009, 61, 288–295. [Google Scholar]
- Gundimeda, U.; Schiffman, J.E.; Chhabra, D.; Wong, J.; Wu, A.; Gopalakrishna, R. Locally generated methylseleninic acid induces specific inactivation of protein kinase C isoenzymes: Relevance to selenium-induced apoptosis in prostate cancer cells. J. Biol. Chem 2008, 283, 34519–34531. [Google Scholar]
- Zuo, L.; Li, J.; Yang, Y.; Wang, X.; Shen, T.; Xu, C.M.; Zhang, Z.N. Sodium selenite induces apoptosis in acute promyelocytic leukemia-derived NB4 cells by a caspase-3-dependent mechanism and a redox pathway different from that of arsenic trioxide. Ann. Hematol 2004, 83, 751–758. [Google Scholar]
- Vander-Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar]
- Pérez-Sampietro, M.; Casas, C.; Herrero, E. The AMPK family member Snf1 protects Saccharomyces cerevisiae cells upon glutathione oxidation. PLoS One 2013, 8, e58283. [Google Scholar]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett 2006, 160, 171–177. [Google Scholar]
- Mijatovic, S.; Maksimovic-Ivanic, D.; Radovic, J.; Miljkovic, D.; Kaludjerovic, G.N.; Sabo, T.J.; Trajkovic, V. Aloe emodin decreases the ERK-dependent anticancer activity of cisplatin. Cell. Mol. Life Sci 2005, 62, 1275–1282. [Google Scholar]
- Janjetovic, K.; Vucicevic, L.; Misirkic, M.; Vilimanovich, U.; Tovilovic, G.; Zogovic, N.; Nikolic, Z.; Jovanovic, S.; Bumbasirevic, V.; Trajkovic, V.; et al. Metformin reduces cisplatin-mediated apoptotic death of cancer cells through AMPK-independent activation of Akt. Eur. J. Pharmacol 2011, 651, 41–50. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, J.; Li, J.; Zhang, J.-F.; Xin, X.-Y. Combination of Fenretinide and Selenite Inhibits Proliferation and Induces Apoptosis in Ovarian Cancer Cells. Int. J. Mol. Sci. 2013, 14, 21790-21804. https://doi.org/10.3390/ijms141121790
Liu J, Li J, Zhang J-F, Xin X-Y. Combination of Fenretinide and Selenite Inhibits Proliferation and Induces Apoptosis in Ovarian Cancer Cells. International Journal of Molecular Sciences. 2013; 14(11):21790-21804. https://doi.org/10.3390/ijms141121790
Chicago/Turabian StyleLiu, Jie, Jia Li, Jian-Fang Zhang, and Xiao-Yan Xin. 2013. "Combination of Fenretinide and Selenite Inhibits Proliferation and Induces Apoptosis in Ovarian Cancer Cells" International Journal of Molecular Sciences 14, no. 11: 21790-21804. https://doi.org/10.3390/ijms141121790



